International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 838
ISSN 2229-5518
Utility of Arylmethylenemalononitriles In Heterocyclic Synthesis : New Synthetic Procedures to Synthesize 4H-pyrano[3,2-c] quinoline, Pyrazolo[4,3-b] pyridine, 4H -Benzo [b]pyan , Pyridine and [1,3,4]Thiadiazolo[3,2-a] pyridin-2-yl)benzamide Derivatives
aFathyMuhammad AbdelAziz El-Taweel*, bTarek Mohamed
AboElMaati and aMuhammad Mofeed1
aDepartment of Chemistry , Faculty of Science , New Damietta City, Damietta University,Damietta,34517,Egypt
bDepartment of Chemistry , Faculty of Applied Arts , Damietta City, Damietta University,Egypt
ABSTRACT- 4H-pyrano[3,2-c]quinolines 7a,b were prepared via
reacting arylmethylenemalononitrile 1a with 3-acetyl-4-hydroxyquinoline
2 or 4-hydroxyquinoline 3.Pyrazolo[4,3-b]pyridines 11a-f were obtained by reacting 1a-f with 4-nitrosoantipyrine 8.Reaction of 1g with dimedone
12 and the hydrazone 15 resulted in the formation of 4H-benzo[b]pyran
14 and pyridine 19 respectively. Compound 1 reacted with 1,3,4- thiadiazole 20 to afford [1,3,4]thiadiazolo[3,2-a]pyridin-2-yl) benzamides23.
Keywords:Arylmethylenemalononitriles,4-hydroxyquinolines ,4H-
pyrano[3,2-c]quinolnes, pyrazolo[4,3-b]pyridines,pyridine, 4H- benzo[b]pyran , [1,3,4]thiadiazolo[3,2-a]pyridin-2-yl)benzamides
*Corresponding Author (E-mail: fathyeltaweel@ yahoo.com ; Tel .:
+201278835201; Fax:+2057403868. Abstracted from his M.Sc.Thesis.

1 Introduction
Arylmethylenemalononitriles are versatile reagents which react with
nucleophiles under mild conditions[1-4]. In the past decade, we were involved in a program aimed at developing the synthesis of polyfunctionally substituted heterocycles as potential biodegradable agrochemicals [1,2] and antischistosomal agents[5-11]. During this phase of our research, we have been investigated the base catatlysed reactions
of cinnamonitriles with active hydrogen reagents.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 839
ISSN 2229-5518
In connection to this effort, we report here new approach for synthesis of polyfunctionally substituted 4H-pyrano[3,2-c]quinoline ,pyrazol[4,3-b] pyridines ,4H-benzo[b]pyran and pyridine derivatives ,that have been extensively studied due to their commercial applications in several fields[1-11].
2 EXPERIMENTAL
All melting points are uncorrected and measured on Griffin George MBF 010T (London) apparatus. Recorded yield correspond to the pure products. IR (KBr) spectra were recorded on a Perkin Elmer SP-880 spectrometer and 1H-NMR spectra: were measured on Varian 270 MHz spectrometer on DMSO-dR6 Ras solvent and TMS an internal standard. Chemical shifts are reported in δ units (ppm). Microanalyses were performed on a LECO CHN-932 elemental analyzer and carried out in the Microanalytical Data Unit at Cairo and Damietta Universities. Mass spectra were recorded on a MS 30(AEI) instrument at 70 eV ionization energy .
Synthesis of pyrano[3,2-c]quinoline derivatives 7a,b:General procedure :
Method A:
A solution of 3-acetyl-4-hydroxy-2(1H)quinolinones 2a,b (0.0 mole) and
(0.0 mole) of 2-(3,4-dimethoxybenzylidene)malononitrile 1a in ethanol (
50 mL) containing few drops of piperidine were refluxed for 15 minutes and then left to cool .The obtained precipitates were collected by filtration and recrystallised from the proper solvents and the identified as 7a,b. Method B:
Copmounds 7a,b were also prepared from 4-hydroxy-2(1H)quinolinones
3a,b (0.01 mole ) and (0.01 mole ) of 2-(3,4- dimethoxybenzylidene)malononitrile 1a utilizing the above reaction conditions.
2-Amino-4-(3,4-dimethoxyphenyl)-6-methyl-5-oxo- 5,6-dihydro-4H- pyrano[3,2-c] quinoline-3-carbonitrile 7a: Formed colorless crystals in
70 % yield , from ethanol / dimethylformamide ,m.p.253-255oC ; IR
(ν/cm-1 ): 3321, 3194(NH
), 2187(conjugated CN),1672(CO); 1H-NMR
R2R
(DMSO-dR6R )(δ,ppm):3.41 (s,3H, N-CHR3R ) ,3.69(s,3H,OCHR3R),3.71(s,3H , OCHR3R), 4.49 (s,1H ,pyran H-4),6.66-6.68 (m, 3H,aromatic protons ),
7.21 (s, 2H,NHR2R)7.39-8.03(m,7H, aromatic protons ). Anal.Calcd.for
CR22RHR19RNR3ROR4R (389.40): C,67.86; H, 4.92; N , 10.79.Found: C,67.67;H,4.76;N,10.62.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 840
ISSN 2229-5518
2-Amino -4-(3,4-dimethoxyphenyl) -6-ethyl-5-oxo--5,6-dihydro-4H- pyrano[3,2-c] quinoline-3-carbonitrile 7b : Formed colorless crystals in
75 % yield , from ethanol ,m.p.237-239oC ; IR (ν /cm-1 ): 3483, 3332
(NH2 ) , 2210(conjugated CN),1631(CO); 1H-NMR (DMSO-d6 )(δ,ppm):
1.19-1.24 (t,J = 7Hz,3H,CH3 ) ,3.70(s,3H,OCH3 ),3.87(s,3H,OCH3 ),4.26-
4.29(q,J = 7Hz,2H,CH2 ) ,4.5.0 (s,1H,pyranH-4),7.14-8.07 (m,10H, aromatic protons ) ,8.82 (s,2H, NH2 ).Anal.Calcd.for C23 H21N3O4 (403.15): C,68.47; H, 5.25; N , 10.42.Found: C,68.60 ; H,5.36; N,11.33.
Formation of 6-aryl-1-methyl-3-oxo-2-phenyl-2,3-dihydro-1H- pyrazolo[4,3-b] pyridine -5-carbonitriles 11 a-f :
A mixture of arylmethylenemalononitriles 1 (0.01mole) and 1,5- dimethyl-4-nitroso-2-penyl-1H-pyrazol-(3(2H)-one 8(0.01mole) in ethanol (50ml),containing few drops of piperidine were refluxed for three hours .The formed solid products were collected by filtration
,recrystallised from the suitable solvents and then identified as 11 a-f .
6-(3,4-Dimethoxyphenyl)-1-methyl-3-oxo-2-phenyl-2,3-dihydro-1H- pyrazolo[4,3-b]-5-carbonitrile 11 a : Formed orange crystals in 70 % yield , from ethanol / dimethylformamide,m.p.224-226oC ; IR (ν /cm-1 ):
2225(conjugated CN),1704(CO); 1H-NMR (DMSO-d6 )(δ,ppm): 3.30
) ,3.76(s,3H,OCH ) ,3.87 (s,3H,OCH ), 7.18-7.64 (m,8H, aromatic protons ),8.42(s,1H, pyridine H-4) . Anal . Calcd . for C22H18N4O3 (403.15): C,68.38; H, 4.70; N , 14.50.Found: C,68.60 ; H,4.36; N,14.33.
1-Methyl-3-oxo-6-(3-phenoxyphenyl)-2-phenyl-2,3-dihydro-1H-pyrazolo [4,3-b]pyridine-5-carbonitrile 11 b : Formed pale yellow crystals in 73 % yield , from ethanol / dimethylformamide,m.p.244-246oC ; IR (ν /cm-1 ):
2229(conjugated CN),1689 (CO); 1H-NMR (DMSO-d6)(δ,ppm): 3.34
(s,3H,N-CH3 ), 7.06-7.67 (m,8H, aromatic protons ),8.48(s,1H, pyridine
H-4) . Anal . Calcd . for C26 H18N4O2 (418.45): C,74.63; H, 4.34; N ,
13.39.Found: C,74.70 ; H,4.26; N,13.33.
6-(4-Hydroxy-3-methoxyphenyl)-1-methyl-3-oxo-2-phenyl-2,3-dihydro-
1H-pyrazolo[4,3-b]-5-carbonitrile 11 c : Formed red crystals in 65 % yield , from ethanol,m.p.260-262oC ; IR (ν /cm-1 ): 2224(conjugated CN),1661 (CO); 1H-NMR (DMSO-d6)(δ,ppm): 3.38 (s,3H,N-CH3) ,3.87
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 841
ISSN 2229-5518
(s,3H,OCH3 ), 6.97-7.59 (m,8H, aromatic protons ),8.37(s,1H, pyridine H-
4) ,9.94(s,1H,OH) ; 13C-NMR (DMSO-d6)(δ,ppm):38.02(N-CH3),
56.32(OCH3 ),113.89-148.77(aromatic carbons), 118.79(CN), 158.42 (CO) . Anal . Calcd . for C21 H16N4O3 (372.38): C,67.73; H, 4.33; N ,
15.05.Found: C,67.83 ; H,4.26; N,15.40.
6-(3-Chlorophenyl)-1-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazolo [4,3-b]-5-carbonitrile 11 d : Formed yellow crystals in 60 % yield , from ethanol / dimethylformamide,m.p.246-248oC ; IR (ν /cm-1 ): 2228 (conjugated CN),1695 (CO); 1H-NMR (DMSO-d6 )(δ,ppm): 3.31 (s,3H,N- CH3 ) , 7.47-7.85 (m,10H, aromatic protons ),8.51(s,1H, pyridine H-4) . Anal . Calcd . for C20 H13ClN4O (360.80): C,66.58; H, 3.63; N ,
15.53.Found: C,66.83 ; H,3.44; N,15.42.
1-Methyl-6-(4-nitrophenyl)-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazolo [4,3-b]-5-carbonitrile 11 e : Formed orange crystals in 65 % yield , from ethanol / dimethylformamide,m.p.>300oC ; IR (ν /cm-1 ): 2225 (conjugated CN),1701 (CO); 1H-NMR (DMSO-d6 )(δ,ppm): 3.31 (s,3H,
N-CH3) , 7.47-7.54 (m,5H, aromatic protons ),8.03-8.06 (d,J=7Hz, 2H,
aromatic protons),8.46-8.49(d,J=7Hz,2H,aromatic protons), 8.56 (s,1H, pyridine H-4) . Anal . Calcd . for C20 H13ClN4O (360.80): C,66.58; H,
3.63; N , 15.53.Found: C,66.83 ; H,3.44; N,15.42.
1-Methyl-6-(4-nitrophenyl)-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazolo [4,3-b]-5-carbonitrile 11 f : Formed yellow crystals in 80 % yield , from ethanol / dimethylformamide,m.p.>300oC ; IR (ν /cm-1 ): 2225 (conjugated CN),1691 (CO); 1H-NMR (DMSO-d6 )(δ,ppm): 3.35 (s,3H,
N-CH3) , 6.97-7.59 (m,7H, aromatic protons ), 8.37 (s,1H, pyridine H-4) .
Anal . Calcd . for C18 H11 BrN4 OS (411.28): C,52.57; H, 2.70; N ,
13.62.Found: C,52.46 ; H,2.54; N,13.42.
Preparation of 2-amino-7,7-dimethyl-4-(4-nitro-1H-pyrrol-2-yl)-5-oxo-
5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 14:
A suspension of 2-((4-nitro-1H-pyrrol-2-yl)methylene)malononitrile
1g(0.01mole) in ethanol (50ml) containing (0.01mole) of 5,5- dimethylcyclohexane-1,3-dione ,was treated with few drops of triethylamine and heated under reflux for five hours .The precipitate formed was collected by filtration ,recrystallised from ethanol as colorless
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 842
ISSN 2229-5518
crystals,in 60% yield,m.p.218-220oC; IR (ν /cm-1 ):
3481,3326(NH2 ),2196 (conjugated CN); 1H-NMR (DMSO-d6 )(δ,ppm):
0.98(s,3H,CH3 ), 1.02 (s,3H,CH3),
2.18(s,2H,CH2 ),3.18(s,2H,CH2 ),4.28(s,1H,pyran H-4),6.31-6.32(t,J=
7Hz,1H,aromatic proton),7.06(s,2H,NH2), 7.70-7.72(t,J= 7Hz,
1H,aromatic proton),11.92(s,1H,NH). Anal . Calcd . for C16H16N4 O4
(328.32): C,58.53; H, 4.91; N , 17.06.Found: C,58.65 ; H,4.74; N,17.12.
Formation of 6-amino -4-(4-nitro-1H-pyrrol-2-yl)-2oxo-1-((-thiophen-2- yl)ethylidene)amino)-1,2-dihydropyridine-3,5-dicarbonitrile 19 :
A mixture of 2-cyano-N,-(1-(thiophene-2-yl)ethylidene)acetohydrazide
15(0.01mole) and 2-((4-nitro-1H-pyrrol-2-yl)methylene)malononitrile 1g (0.01mole) in ethanol (50ml) containing catalytic amounts of piperidine was refluxed for three hours .The precipitate formed was collected by filtration ,recrystallised from ethanol / dimethylformamide as yellow crystals,in 60% yield,m.p.248-250oC; IR (ν /cm-1 ): 3447,3150
(NH2, NH),2211 (conjugated CN),1658(CO); 1H-NMR (DMSO-d6 )
(δ,ppm) : 2.42 (s,3H,CH3), 7.09-7.63(m,7H,aromatic proton)
,8.34(s,2H,NH2 ),8.35(s,1H,NH), 12.92(s,1H,NH). Anal . Calcd . for C H N O S (393.38): C,51.90; H, 2.82; N , 24.92.Found: C,51.78 ; H,2.76; N,24.87.
Condensation of N-(5-cyanomethyl)-1,3,4-thiadiazol-2-yl)benzaminde
20 with aromatic aldehydes:Formation of 2-(4-aryl)vinyl-N-[5-1-cyano) -
1,3,4-thiadiazol-2-yl)benzaminde 21:
Compound 20 (0.01mole) in ethanol(50ml) was treated with (0.01mole) of aromatic aldehydes and few drops of piperidine.The reaction mixture was refluxed for two hours . The solids formed were collected by filtration and purified by recrystallization from the proper solvents then identified as 21a,b.
(E)-N-(5-(1-cyano-2-(3,4-dimethoxyphenyl)vinyl)-1,3,4-2-yl)benzamide
21 a : Formed yellow crystals in 70 % yield , from ethanol / dimethylformamide, m.p. > 300oC ; IR (ν /cm-1 ):3419(NH), 2219 (conjugated CN),1653 (CO); 1H-NMR (DMSO-d6 )(δ,ppm): 3.83 (s,3H, OCH3) , 3.86 (s,3H, OCH3 ) , 7.16-8.15 (m,8H, 7H aromatic protons and
1H CH ), 13.29 (s,1H, NH) . Anal . Calcd . for C20H16N4 O3 S (392.43): C,61.21; H, 4.11; N , 14.28.Found: C,61.35 ; H,4.23; N,14.40.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 843
ISSN 2229-5518
(E)-N-(5-(1-cyano-2-(3-phenoxyphenyl)vinyl)-1,3,4-2-yl)benzamide 21 b : Formed yellow crystals in 70 % yield , from ethanol / dimethylformamide,m.p. > 300oC ; IR (ν /cm-1 ):3406(NH), 2225 (conjugated CN),1631 (CO); 1H-NMR (DMSO-d6 )(δ,ppm): 7.11-8.20 (m,8H, aromatic protons ), 9.85 (brs,1H, NH) . Anal . Calcd . for C24H16N4O2 S (424.47): C,67.91; H, 3.80; N , 13.20.Found: C,67.74 ; H,3.65; N,13.40.
Formation of N-(6,8-dicyano-7-(aryl)-5-imino-5H-[1,3,4]thiadiazolo[3,2-
a] pyridin-2-yl)benzamides 23 a-c : Method A:
Equimolecular amounts of 20 (0.01mole) and the appropriate amounts
of arylmethylenemalononitriles 1a,b,f (0.01mole) in absolute ethanol (50ml)and catalytic amount of piperidine were refluxed for three hours.The solid products so formed were filtered off ,recrystallised and then identified as 23 a-c.
Method A:
Compounds 23 a-c were also prepared by reacting equimolecular amounts of 21and malononitrile using the above reaction conditions.
N-(6,8-dicyano-7-(3,4-dimethoxyphenyl)-5-imino-5H-[1,3,4]thiadiazolo [3,2-a]pyridin-2-yl)benzamide 23 a : Formed yellow crystals in 73 % yield , from ethanol / dimethylformamide,m. p.162-164oC ; IR (ν /cm-1 )
:3423(NH), 2215 (conjugated CN),1652 (CO); 1H-NMR (DMSO-
d6)(δ,ppm): 3.83(s,3H,OCH3 ), 3.83(s,3H,OCH3 ),7.14-8.15 (m,9H, 8H aromatic protons and 1H,NH ), 13.23 (s,1H, NH) . Anal . Calcd . for C24H20N6O3 S (472.13): C,67.91; H, 3.80; N , 13.20.Found: C,67.74 ; H,3.65; N,13.40.
N-(6,8-dicyano-7-(3-phenoxyphenyl)-5-imino-5H-[1,3,4]thiadiazolo [3,2- a]pyridin-2-yl)benzamide 23 b : Formed orange crystals in 73 % yield , from ethanol / dimethylformamide,m. p.167-169oC ; IR (ν /cm-1 )
:3423(NH), 2227 (conjugated CN),1659 (CO); 1H-NMR (DMSO-
d6)(δ,ppm): 7.09-8.15 (m,13H, aromatic protons), 8.29(s,1H,NH),13.36 (s,1H, NH) . Anal . Calcd . for C27H16N6 O2S (488.52): C,66.38; H, 3.30; N , 17.20.Found: C,66.46 ; H,3.54; N,17.40.
N-(6,8-dicyano-7-(5-bromothiophen-2-yl)-5-imino-5H-[1,3,4]thiadiazolo
[3,2-a] pyridin-2-yl)benzamide 23 c : Formed yellow crystals in 73 %
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 844
ISSN 2229-5518
yield , from ethanol / dimethylformamide,m. p. > 300oC ; IR (ν /cm-1 )
:3447(NH), 2220 (conjugated CN),1623 (CO); 1H-NMR (DMSO-d6 ) (δ,ppm): 7.46-7.73 (m,6H ,5H, aromatic protons and 1H ,NH),8.11-
8.13(d,J =7Hz , 2H , aromatic protons), 8.48(s,1H,NH); 13C-NMR
(DMSO-d6 ) (δ,ppm): 99.52,121.02, 128.96,129.17,132.30, 133.65,
138.46,138.64,140.07,143.81(aromatic carbons),116.22(CN), 166.04 (C=NH),190.24(C=O). Anal . Calcd . for C19H9 BrN6OS2 (481.25): C,47.41; H, 1.88; N , 17.46.Found: C,47.65 ; H,2.02; N,17.35.
3.RESULTS AND DISCUSSIN
It has been found that ,the reaction of 3-acetyl-4-hydroxyquinolin-
2(1H)-ones 2a,b with arylmethylenenitriles 1a in ethanol and in the presence of catalytic amounts of piperidine ,resulted in the formation ,2- amino-4-aryl-6-(4-hydroxy-2-oxo-1,2-dihydroquinolin-yl)-3-substituuted
-4H-pyran derivatives 4 and 2-amino-4-aryl-5-oxo-5,6-dihydro-4H-
pyrano[3,2-c]quinoline derivatives 7. Structures 4 were readily ruled out by analytical and spectral data of the reaction products.Thus, structures 7 were established for the reaction products based on 1H-NMR spectra which revealed the presence of pyran-4H protons at δ = 4.5-5.0 ppm. Compounds 7 were assumed to be formed via addition of quinolinyl C-3 to the π-deficient center in 2 to give the adduct 5 , which hyrolysed and readily eliminate its acetyl group under the reaction conditions to give the
intermediates 6.These were cyclised to 7.Elimination of the acetyl groups in this reactions parallels the reported deacetylation of similar systems under similar conditions [1,2]. Compounds 2 may be existing as 4- quinolone[1,2] , at which quinolin-3-position becomes more acidic than
its acetyl group.Moreover,the steric effect in the intermediates 5 facilitate deacetylation process .The structures of compounds 7 were also confirmed by synthesizing them from reaction of 4-hydroxyquinolin-
2(1H)-ones 3a,b under the same reaction conditions(c.f.Scheme 1).
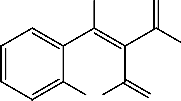
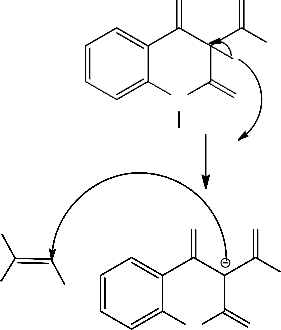
Also, we have found that, arylidenemalononitriles 1a-f reacted readily with 1,2-dihydro-2,3-dimethyl-4-nitroso-1-phenylpyrazol-5-one 8 to give products via hydrogen cyanide and water elimination. 6-Aryl-1-methyl-3- oxo-1,2,3-trihydro-2-phenylpyrazolo[4,3-b]pyridine-5-carbonitriles 11a-f structures were assigned as reaction products based on their elemental analysis and spectral data. Also, IR spectra of 11a-f showed absorption bands corresponding to the cyano and carbonyl groups of phenazonyl moieties. Compounds 11 were assumed to be formed via addition of the
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 845
ISSN 2229-5518
methyl group in 8 to the activated double bond in 1 to give the adducts 9 which cyclized to give the intermediates 10. The later aromatized through elimination of hydrogen cyanide or ethyl formate and water to give11 . Similar sequence for the formation of similar systems has been reported before [2] (c.f. Scheme 2).
In addition ,5,5-dimethylcyclohexan-1,3-dione 12 reacted with 2-((4- nitro-1H-pyrrol-2-yl)methylene)malononitrile 1g in ethanolic / triethylamine to afford 1:1 adduct .This adduct was formulated as 2- amino-7,7-dimethyl-4-(4-nitro-1H-pyrryl-2-yl)-5-oxo-5,6,7,8-tetrahydro-
4H-chromene-3-carbonitrile 14.
2-Cyano-N,-(1-(thiophene-2-yl)ethylidene)acetohydrazide 15 reacted with 2-((4-nitro-1H-pyrrol-2-yl)methylene)malononitrile 1g in ethanol catalysed by piperidine to give either 2-cyano-N-(3-cyano-2-imino-5- methyl-6-(4-nitro-1H-pyrrol-2-yl)-4-(thiophen-2-yl)pyridine-1(2H)- yl)acetamide 17 or 6-amino-4-(4-nitro-1H-pyrrol-2-yl)-2-oxo-1-((1- thiophen-2-yl)ethylidene)amino)-1,2-dihydropyridine-3,5-dicarbonitrile
19.Structure 17 was excluded by 1H-NMR spectrum which clearly
indicates the absence of methylene protons at δ = 4.5 ppm.Thus, structure
19 was was elucidated as a reaction product from its analytical and spectral data(c.f.experimantal).Compound 19 was suggested to be obtained via addition of the activated the active methylene group in 15 to the pi-deficient carbon in 2-((4-nitro-1H-pyrrol-2-yl)methylene) malononitrile 1g to give the adduct 18 which cyclised and dehydrogenated to give 19 (c.f.scheme 3).
We have also investigated the reactivity of arylmethylenemalononitriles 1 towards alkylheterocycles .Thus,we have found that, arylmethylenemalononitriles 1a,b,f reacted readily with N-(5- (cyanomethyl)-1,3,4-thiadiazol-2-yl)benzamide 20 in ethanolic / piperidine to yield either N-(6,8-dicyano-7-(aryl)-5-imino-5H-[1,3,4] thiadiazolo[3,2-a]pyridin-2-yl)benzamides 23 or N-(5-amino-7-aryl-6,8- dicyano-7H-[1,3,4]thiadizolo[3,2-a]pyridine-2-yl)benzamides 24. 1H- NMR spectra of the reaction products revelead no signals at δ ≈ 4.5-5.0 ppm for one proton linked to sp3 carbon corresponding to pyridine H-4 protons.Consequently,Structures 23 were elucidated as reaction products.The formation of 23 was assumed to proceed via Michael type addition of the active methylene group in N-(5-(cyanomethyl)-1,3,4- thiadiazol-2-yl)benzamide 20 to the activated double bonds in the arylmethylenemalononitriles 1 to give Michael adducts 22 ,which readily cyclised and dehydrogenated to afford the final isolable products 23 (c.f.scheme 4).
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 846
ISSN 2229-5518
CN

Ar CN

1 Ar
a 3,4-di-OCH3.C6H3
b 3-OPh.C6H4
c 4OH,3-OCH3.C6H3
d 3-Cl.C6H4
e 4-NO2.C6H4
f 5-bromo-2-thienyl
4-nitro-2- rr l
IJSER
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 847
ISSN 2229-5518

OH
COCH3
OH O

NH2
CN
Ar

N O N O R R


2 4
NC
O CN
R3 CN
Ar
COCH3
N O
R
5


 H2O
H2O  CN
CN
- CH3CO2H

NC
1 OH CN
Ar
IJSER

N O
R1
6
OH
N O O R
3

N
R

NH2
CN
Ar
O


7 R Ar
a
CH3
3,4-di-OCH3.C6H3
Scheme 1:Formation of 4H-pyrano[3,2-c]quinolines 7
b C2H5 3,4-di-OCH3.C6H3
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 848
ISSN 2229-5518
OH O O O

CH3
CH3
H
N O N
 2
2
R R
-pip.H
O
pip.
NC O O

NC 1 Ar
CH3


NC CN O

N O
R

NC CN
O
IJSER
Ar
COCH3
N O R

5
OH
H2O
-CH3CO2H
CN
Ar
N O R
6
NH2
CN O

Ar 1 CN Ar

N O
R N R
7 
R
Mechanim f or f ormation of compounds 7
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 849
ISSN 2229-5518


Ar CN H3C NO
1

CN
N

H3C N O
Ph
8

CN
Ar
CN NO
N

H3C N O
Ph
9
Ar CN

Ar CN

CN N
N OH
IJS-HCNER
N

H3C N
Ph
-H2O O
N

H3C N O
Ph
11 Ar 10
a 3,4-di-OCH3.C6H3
b 3-OPh.C6H3
c 4-OH,3-OCH3.C6H3
d 3-Cl.C6H4
e 4-NO2.C6H4
f 5-bromo-2-thienyl
Scheme 2 :Formation of pyrazolo[4,3-b]pyridines 11
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 850
ISSN 2229-5518

O


O Ar
CN
12 O
CN
13 OH
O Ar
CN CN
Ar CN
1
O NH2
NNHCOCH2CN
14,Ar =4-nitro-2-pyrryl
R, = CH3
R 15 R,
O CN

NC ICN JSER
NHNHCOCH2CN
Ar R
16
-H2
R
Ar
NC R,
18 CN
-H2

O
NC
NNHCOCH2CN

Ar
NC CN
Ar R
17

H2N N O
N

R R,
19, R=2-thienyl,R, = CH3 Ar =4-nitro-2-pyrryl
Scheme 3 :Formation of benzo[b]pyrans 14 and pyridine 19
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 851
ISSN 2229-5518
N N
CN
R
S


20, R = PhCONH-
Ar 1
R

CN
CN
NC N NH
S
CN NC CN R
Ar
ArCHO
N N
CN S
Ar
22 CN
21a,Ar = 3,4-di.OCH3.C6H3
b,Ar = 3-OPh.C6H4

I-H2 JSER
HN H2N
CN CN

N N N N
Ar Ar
R S R S
CN 24 CN
23a , Ar = 3,4-di.OCH3.C6H3
b,Ar =3-PhO.C6H4
c,Ar = 5-bromo-2-thienyl
Scheme 4 : Formation of 1,3,4-thiadiazolo[2,3-b]pyridines 23
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 852
ISSN 2229-5518
Conclusion
We conclude that,several new 4H-pyrano[3,2-c]quinoline,pyrazolo[4,3-b] pyridine,4H-benzo[b]pyran , pyridine and thiadiazolo[3,2-a]pyridine derivatives were prepared via reacting active hydrogen reagents with arylmethylenemalononitriles as readily obtainable starting materials that could be useful for biological evaluation studies.
REFERENCES
[1] El-Taweel, F.M., Elagamey, A.A. and Khalil M.H.,"Studies on quinoline-2(1H)-one," Am.Chem.Sci.J., 2013,3,4,532-549 .
[2] El-Taweel F.M.A.and Elagamey A.A.," New and facile synthesis of substituted heterocycles," Int.J.Org.Chem.,2013, 3, 58-70.
[3] Mandour, A.H.M., El-Sawy,R., Ebaid,M.S. and Hassan,
S.M."Synthesis and potential biological activity of some novel 3-[(N- substituted indol-3-yl)methyleneamino]-6-amino-4-arylpyrano[2,3-c] pyrazole-5-carbonitriles," Acta Pharm.,2012, 62, 15-30.

[4] Mphahlele,M.J.,''Synthesis of 2-Arylquinolin-4(1H)-ones and Their Transformation to N-alkylated and O-alkylated Derivatives,' 'J. Heterocycl.Chem.,2010, 47,1-14.doi:10.1002/jhet.279.
[5] Abass , A., Mohamed ,E.A., IsmailM.M. and Mayas,A.S.,
"Substituted Quinolone.Part 16.preparation and Reactions of 3-(4- hydroxy-1-methyl-2-oxo-1,2-dihydroquinolin-3-yl)-3-oxopropanoic acid," Eur.J. Chem., 2011, 2, 3, 378-387. doi:10.5155/eurjchem.2.3.378.351.
[6] Kozlov,N.G., Gusak K.N.and Kadutskii,A.P.,"Development of the Catalytic Synthesis of Compounds of the Quinoline Series,"Chem.of Heterocycl. Compounds ," 2010, 46,, 5, 505-528.
[7] Hassanin H.M.and Abdel-Kader ,D.,"Synthesis of Some Novel
binuclear Heterocyclic Compounds From 6-Ethyl-3-nitropyrano[3,2-c]
quinolin-4,5(6H)-dione,Heterocycles," 2013,,87, 2, 369-380. doi: 10.
3987/COM-12-12639.
[8] El-Agrody, A.M.and Al-Ghamdi ,A.M.,"Synthesis of Certain Novel
4H-pyrano[3,2-h]quinoline Derivatives," Arkivoc , 2011, xi, 134-136, .
[9] Ibrahim,M.A., Hassanin ,H.M., Gabr Y.A.and Alnamer ,Y.A."Studies on the Chemical Behavior of 3-(Nitroacetyl)-1-ethyl-4-hydroxyquinolin-
2(1H)-one Towards Some Electrophilic and Nucleophilic Reagents, "J.Braz .Chem. Soc., 2012, 23, 5, 905-912.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 853
ISSN 2229-5518
[10] Hassanin, H.M.,"Nucleophilic Substitution and Ring Transformation Reactions with 4-Chloro-6-ethyl-3-nitropyrano[3,2- c]quinoline-2,5-(6H)-diones, " Arkivoc, 2012, vi,384-397.
[11] Rufchahiam M.and Gilani,A.G.,"Synthesis ,Characterization and Spectroscopic Properties of Some New Disperse Dyes Derived From 4- hydroxybenzo[h]quinolin-2-(1H)-one as a new Synthesized Enol Type Coupling Component ,"Dyes and Pigments ,2012, 95, 3, 632-636.
IJSER
IJSER © 2014 http://www.ijser.org