International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 444
ISSN 2229-5518
Thermodynamic Analysis and Comparison for “COP” In Solid Adsorption Refrigeration Systems (BaCl2-NH3 & SrCl2-NH3)
Jitendra Kumar, Naushad.A.Ansari, Vikas Verma , Pradhuman Dobhal
Abstract-Solid sorption refrigeration systems are environment friendly as they use low grade thermal energy and the refrigerants used have zero ozone depletion potential and very low global warming potential. Though lot of efforts have been made in this field, COP of the solid sorption refrigeration system is still quite low compared to vapor compression refrigeration systems, thus there is a need for further research to make these systems a viable alternative to vapor compression systems. In this paper solid BaCl2 and SrCl 2 are used as solid absorbent and ammonia as refrigerant .The technical feasibility of the system is studied using a simple thermodynamic model assuming ideal material kinetics. In this paper it shown that the variation of the COP & second law COPC with the variations of evaporator temperature, Ratio of mass of reactor material to that of absorbent bed mr and maximum desorption temperature .The main results showed that the generation temperature is very low for the BaCl2 - NH3 system, as shown by a comparative study of SrCl2 - NH 3 system and BaCl 2 - NH3 systems.
Index term- COP, Second law COP C , BaCl2 , SrCl2 , NH3 , mr
—————————— ——————————
Due to ongoing energy crises people are thinking about greater use of renewable energy sources. In many regions of developing countries refrigeration is required for food and medicine preservation, as well as ice production for the development of fishing communities. Due to their economic conditions, simple refrigeration units are required which can operate with thermal energy, when electric supply is faulty and/or expensive. The adapted thermal refrigeration cycles are those of absorption, adsorption and the thermo-chemical (solid-gas) absorption. Adsorption refrigeration system uses solid adsorbent beds to adsorb and desorbs a refrigerant to obtain cooling effect. These solid adsorbent beds adsorb and desorb a refrigerant vapour in response to changes in the temperature of the adsorbent.![]()
• Naushad A. Ansari is an Asst. Prof. in Department of Mechanical
Engineering, Delhi Technological University, India PH-+919818243128
• Vikas Verma Phd Scholar Metallurgical and Materials Engineering IIT Roorkee
India PH- 9412448619. E-mail: vikasverma.iitr@rediffmail.com
• Pradhuman dobhal Asst. Professor in Shivalik College of Engineering,
Dehradun Uttarakhand, India, PH- 8979560172. E-mail:pradhuman.dobhal@ymail.com
An adsorption refrigeration circuit usually consists of four main components, solid adsorbent bed, condenser, throttling valve and an evaporator. Whole of the system is shown in figure 1.1.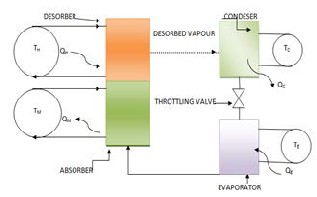
Fig1. Basic adsorption system
In this system the solid adsorbent bed desorbs refrigerant when heated and adsorb refrigerant vapour when cooled. In this way, the bed is used as a thermal compressor to drive the refrigerant around the system to heat or cool a heat transfer fluid or to provide space heating or cooling. The refrigerant is desorbed from the bed as it is heated to drive the refrigerant out of the bed and the refrigerant vapour is moved to a condenser. In the condenser, the refrigerant vapour is cooled and condensed to liquid, and then the refrigerant condensate expands to a lower pressure evaporator where the low pressure condensate is heat exchanged with the space to be conditioned to vaporize the condensate. When further heating no longer produces desorbed refrigerant from the adsorbent bed, the bed is isolated and allowed to return to the adsorption conditions. When the adsorption conditions are established in the bed, the refrigerant vapour from the evaporator is reintroduced to the bed to complete the cycle. For the adsorption system furthermore improvement in the COP different adsorption working pairs are used, such as,CaCl2 -NH3 , MnCl2 -NH3 ,Silica gel/water, zeolite/water after this novel adsorption systems are invented with different working pairs. For
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 445
ISSN 2229-5518
one bed machine, simple structure and low cost are pursued. Several multi-bed systems are suggested to provide steady refrigeration and higher energy performance.
Li Yong et al [1] This paper reviews more than 100 patents filed mainly since year 2000 that propose technologies to improve adsorption system and make it become a realistic alternative. The patents surveyed are classified into four main groups: adsorption system development, adsorbent bed innovation, adsorbent/adsorbate material development and novel application of adsorption cooling system. The various technology options are discussed and evaluated. “Hot spots” and key inventors/applicants are identified. An assessment is made about current and future development of adsorption refrigeration technologies’. Rivera et al [2] an experimental intermittent thermochemical refrigeration system operating with barium chloride–ammonia reaction is described. The barium chloride is used as solid absorbent and ammonia as refrigerant. The equipment components and the experimental preliminary results are also presented. The main results showed that the generation temperature was
53°C for a condensation temperature of 23°C. In the
evaporation–absorption process, the evaporating temperature was between −10 and 0°C. The results showed the technical feasibility to operate this refrigeration system with low cost solar flat plate collectors in remote areas. L.W. Wang et al [3] Solid sorption refrigeration is a type of environmental benign and energy saving technology and the sorbents utilized can be divided into physical, chemical and composite sorbents, according to the nature of the forces involved in the adsorption process. The types, characteristics, advantages and disadvantages of different adsorbents, refrigerants and working pairs are summarized in this paper, together with the models that describe the adsorption equilibrium. Moreover, some of the procedures to prepare composite adsorbents are presented. The application of different working pairs for different situations is related with the adsorption heat, the adaptability to the driving temperature and to the desired working pressure. The methods to measure the adsorption quantity of different working pairs are compared, and future research directions of adsorption working pairs are also analyzed J.V. Veselovskaya et al [4] a composite adsorbent composed of BaCl2 impregnated into expanded vermiculite has been synthesized and tested in a laboratory scale adsorption chiller. Previous work has established the promising theoretical performance of this adsorbent with ammonia as a refrigerant, in terms of equilibrium uptake, suitable equilibrium temperatures for use in air conditioning applications and good reaction dynamics. Analysis of the adsorption phase revealed a simple exponential approach to equilibrium uptake which was not previously observed in larger scale experiments. It was demonstrated that this material can provide effective operation of the chiller using a low potential heat source (80–90°C) giving COP as high as 0.54 ± 0.01 and SCP ranging from 300 to 680
W/kg. The specific cooling power depends strongly on the driving temperature difference and the cycle duration. Y. Zhong et al [5] Equilibrium concentration characteristics of ammonia with a composite adsorbent material (BaCl2 impregnated into a vermiculite matrix) are investigated: the maximum concentration is about 0.4 kg ammonia/kg adsorbent. Hysteresis was observed between the synthesis and the decomposition reactions. The analysis of the data suggests that the hysteresis could be due to the dimensional changes of the solid during the reactions. The bi-variant behaviour observed was contrary to the mono-variant behaviour anticipated and the reasons are discussed. The COP of a basic adsorption cycle for typical ice-making and air-conditioning applications utilizing ammonia and the composite material were calculated. The results show that the material could be used for air-conditioning or other refrigeration applications. The COP could reach up to 0.6 at typical conditions (T E = 15°C, T C = 35°C).
The working cycles of the system which is based on the figure 1, is shown here for the BaCl2 -NH3 &SrCl2 -NH3 the cycle which is shown in figure 2. is for BaCl2 -NH3 system and the cycle which is shown by the figure 3, is for SrCl2- NH3 system.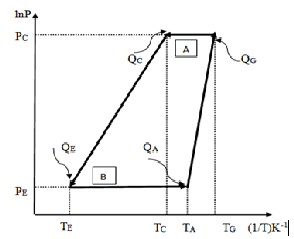
Fig. 2 lnP vs.-1/T diagram for BaCl2 - NH3 system
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 446
ISSN 2229-5518
ln (P×105) = 29.3 - (5012/T)
With the heat of reaction:
∆H=41.7kJmol-1
(6)
(5)
For the ammonia equilibrium the vapour pressure is represented by:
ln (P) = 11.676 − (2793.3/T)
(7)
Where the pressure P is expressed in Pa and temperature
T in K, with a reaction heat.
For the desorption process A as shown in figure 2.1.
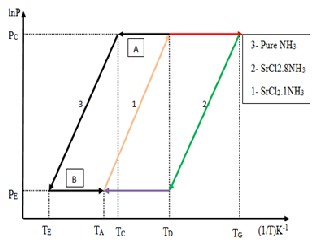
Fig. 3 lnP vs.-1/T diagram for SrCl2 -NH3 system
QG = MNH3 ×∆H + ∑mi C i (T G -T A )
During evaporation.
(8)
From the energy balance equations for a single stage solid
QE = MNH3 × (h2 -h1 ) – CNH3 × (T C -T E ) - MREA ×CREA× (T C -T E )
(9)![]()
![]()
QE MNH3 ×(h2−h1 )−CNH3 ×(TC−TE )−MREA ×CREA ×(TC−TE)
COP= =
absorption refrigeration cycles shown in the figure 2.1
QG +Q E =QC +Q A
(1)
QG MNH3 ×∆H+∑miCi(TG −TA)
(10)
Where QG , QE , QC and QA are the generation, evaporation, condensation and absorption heats respectively. It is possible to establish a relation that will allow the evaluation of the thermal behaviour of the cycle:
QE
The chemical reaction between SrCl2 and ammonia is represented by the following equilibrium relation [6].
SrCl2 . 8NH3 ↔ SrCl2 .1NH3 + 7NH3
(11)
COP =
COPC =![]()
QG
![]()
TE ×
TC −TE
![]()
TG −TC
TG
(2)
(3)
(X) (Y) (Z)
In this case one mole of SrCl2 can absorb 8 moles of NH3 , but generate/desorbs only 7 moles under the design condition of the system. So one mole always remain absorbed in SrCl2 . Referring to the Figure 2.2 the vapor pressure as function of temperature can be represented by
In the present study results from thermodynamic analysis
are obtained for two systems: BaCl2 - NH3
SrCl2 - NH3 .
the following equation with pressure in bar and
temperature in Kelvin (K)[6].
(Equilibrium equation for SrCl2 .8NH3 )
The chemical reaction between barium chloride and ammonia is represented by following equilibrium relation [2]:
BaCl2 .8NH3 ↔ BaCl2 +8NH3
(4)
ln (P) = 15.6229 − (4692.41371/T)
(Equilibrium equation for SrCl2 .1NH3 )
ln (P) = 17.67.67906 − (5786.85423/T)
(12)
(13)
In this case, 8 mole of ammonia for each mole of barium chloride can be recuperated during the dissociation period, in stage one. The vapor pressure as a function of temperature is represented by the Van’t Hoff equation for the equilibrium of a solid gas reaction. With pressure in bar and temperature in Kelvin (K) [2]:
Heat of reaction: ∆H = (46.2251-0.0976(T G -273)) ×58.819
kJ/kg of NH3 (14)
In the above equation T G is the generation temperature expressed in Kelvin (K). Specific heat of composition (X) that is SrCl2 .8NH3 [6] and that of composition (Y) that is SrCl2 .1NH3 [6] are presented by the equations (15) and (16)[6] .
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 447
ISSN 2229-5518
CX = (576.296+ (3.124× (T-273) + 0.07(T-273)2) + 0.00005(T-
273)3)/1000 kJ/kg K (15)
CY = (649.8+ (3.524× (T-273) + 0.021(T-273)2) + 0.00016(T-
273)3)/1000 kJ/kg K (16)
Referring to the figure 2.2 for the process A (desorption).
QE = MNH3 × (h2-h1) – CNH3 × (T C -T E ) - MREA ×CREA × (T C -T E ) (17)
QG = MX ×C X × (T D -T A) + MY ×C Y × (T G -T D ) +MNH3 ×∆H +
MREA×CREA× (T G -T A ) (18)
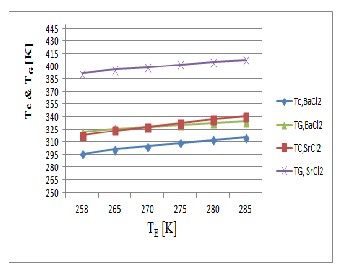
of BaCl2 system. In the figure 4, it clears that the temperature difference (T G -T C ) for SrCl2 is greater than the temperature difference for BaCl2 . So from this figure we come to know that BaCl2 system requires lower heat source temperature compared to SrCl2 system.
COP![]()
=
MNH3 ×(h2 −h1)− CNH3 ×(TC −TE)−MREA×CREA×(TC −TE)
MX×CX×(TD−TA)+MY×CY×(TG−TD)+MNH3 ×∆H+ MREA×CREA×(TG−TA)
(19)
The detailed specification of the ice making chillier for which the thermal analysis of the chillier is being proposed. The Input data for both the system is shown by the table 1.
TABLE 1: INPUT DATA FOR BaCl2 -NH3 & SrCl2 -NH3 SYSTEM
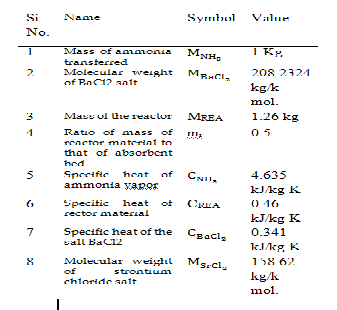
Figure 4, obtained from the result in shows the variation of condenser and generator temperature with evaporator temperature for (mr = 0.5). So from the figure 4. it is clear the variation of the condenser temperature and the generator temperature for the different solid adsorbent salt BaCl2 and SrCl2 .For the same condenser and generator temperature of SrCl2 system is more than that
Fig. 4 Variation of condenser and generator temperature with evaporator temperature
Figure 5, obtained from the result in shows the variation of COP with evaporator temperature for mr =0.5. From the figure it is clear that with increase of evaporator temperature there is increase in COP because with increase of evaporator temperature although there is decrease in (T C - T E ) and (T G - T A) but the reduction in QE = (h2 -h1 ) is more for both the system. From the figure it is clear that for the same evaporator temperature COP of SrCl2 system is more compared to BaCl2 system because for the given range of the evaporator temperature the enthalpy of reaction for SrCl2 system is less than that of BaCl2 system. In case of BaCl2 system COP varies from
0.424788-0.447282 and for SrCl2 it varies from 0.42731-
0.449309 for the given range of T E . It is clear from the figure that BaCl2 , salt yields marginally lower COPs
compared to SrCl2 system and the required heat sink temperatures are also lower for BaCl2 system compared to SrCl2 system. It is preferable to use BaCl2 salt for solid
absorption system because barium chloride salt is cheaper
than SrCl2 salt.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 448
ISSN 2229-5518
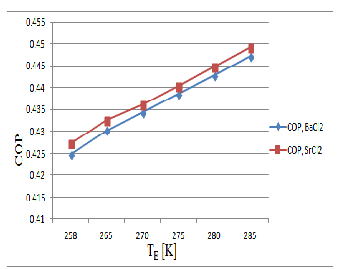
Fig. 5 variation of COP with evaporator temperature
Figure 6, obtained from the result shows the variation of second law COP with evaporator temperature for mr =0.5. From the figure it is clear that for a given evaporator temperature the Second law COP is less for SrCl2 system as ∆T = (T G -T A) is more for the SrCl2 system than that of BaCl2 system. In case of BaCl2 system second law COP varies from 0.55674-0.5569 and for SrCl2 system it varies from 0.0972-0.111796 when T E varies from 258K-285K.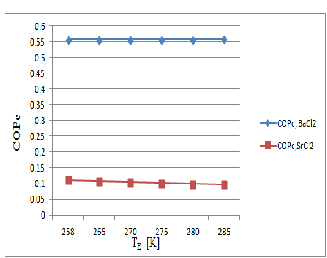
Fig. 6 Variation of second law COP C with evaporator temperature
For extreme climate conditions in tropical areas with ambient temperatures between 30°C and 40°C and for temperatures for ice formation of -10°C, the COP oscillates between 0.40 and 0.45 with minimum temperatures in the desorber between 50°C and 63°C.
Based on the thermodynamic model, coefficient of performance and useful heat for BaCl2 and SrCl2 working
pairs has been calculated for different operating conditions and the results. The COP for both the system will vary same manner. Figure 7 & 8 show that BaCl2 Pair is better than the SrCl2 because BaCl2 decomposed easily
at low temperature (T D = 322.18K) and the SrCl2 starts to decomposed at the higher temperature (T D = 368.26K) when the evaporator maintains at the (T E = 258K).while the COP of BaCl2 is near about the COP of SrCl2 .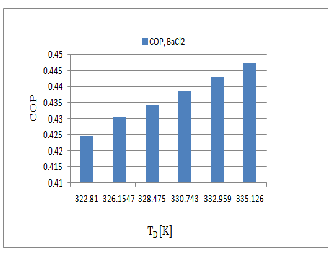
Fig .7 Influence of the maximum desorption temperature on the COP
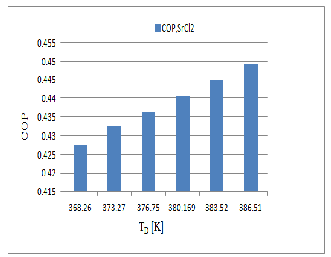
Fig .8 Influence of the maximum desorption temperature on the COP
Figure 9&10 obtained from the result represent the variations of the coefficient of performance in relation to the evaporation and condensation temperature is from
20°C to 40°C, taken every 5°C and the evaporation from
-20°C to 10°C, taken every 5°C. For the BaCl2 salt. It clear from the figure that the COP of the salt will varies linearly
as a straight line corresponding to the different evaporation temperatures. The COP difference for two temperature difference of condenser as 5°C will be almost same from the beginning to the end corresponding to the evaporator temperature from -20°C to 10°C.
In figure 9, it can be observed that COP increase when the
evaporation temperature rises and the condensation
temperature decreases. The maximum value of COP is
0.448 corresponds to T E of 10°C and to T C of 20°C. For the dominion of operation temperature, the COP varies from
.39 to .448. It is possible that the increase of the COP
depends more on T C than on T E .
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 449
ISSN 2229-5518
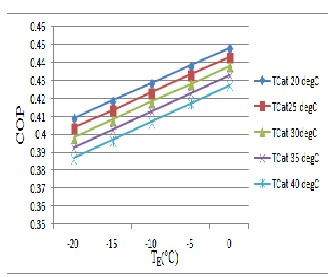
Fig .9 Evolution of the COP as a function of the evaporation temperature
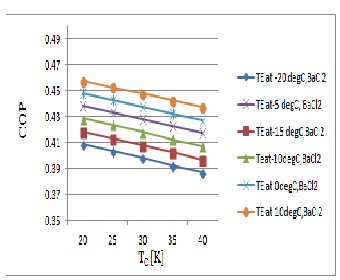
Figure 10, shows that COP decreases when T C increase and T E decreases. In this case COP depends less on T E . For this specific case, COP varied between 0.38 and 0.467.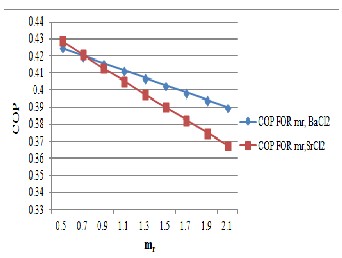
Fig .11 Variation of COP with m r
Based on thermodynamic analysis for the BaCl2 system COP varies from 0.424788-0.447282 and second law COP varies from 0.55674-0.5569 and generator temperature varies from 323K-335K following condenser temperature varies from 296-315K for the range of evaporator temperature from 258K-285K and for SrCl2 system COP varies from 0.42731-0.449309 and second law COP varies from 0.0972-0.111796 generator temperature varies from
391K-407K and desorption temperature varies from 368K-
387K with the condenser temperature variation from
Fig .10 Evolution of the COP as a function of the condenser temperature
Figure 11, shows the variation of COP with mr . from the figure it is clear that with increase of mr there is decrease in COP but it is more sensitive in SrCl2 system because
∆T= T G -T A is more for the SrCl2 system. Figure also shows that the COP difference for both the salt is very
close for mr = 0.5 and the difference varies when the value of mr increases. So mr =0.5 is the more suitable value for both the reactors.
318K-341K for the range of evaporator temperature from
258K-285K.So from the thermodynamic analysis we come to know that BaCl2 system requires lower heat source
temperature compared to SrCl2 system. Also it has higher second law COP. The barium chloride salt is cheaper than the SrCl2 salt. Hence it is preferable to use BaCl2 salt for solid absorption system even though it yields marginally lower COPs compared to SrCl2 system and the required heat sink temperatures are also lower for BaCl2 system compared to SrCl2 system. However, in tropical countries where the ambient temperatures are high, and /or when lower refrigeration temperatures are required for a given heat sink temperature, SrCl2 may be preferable to BaCl2 .
1. [1] Li Yong and Ruzhu Z. Wang Adsorption Refrigeration: A Survey of Novel Technologies Recent Patents On Engineering,
1(2007), 1-21
2. [2] C. Rivera, I. Pilatowsky, E. Mendez, W. Rivera Experimental study of a thermochemical Refrigerator using the barium chloride ammonia reaction. International journal of Hydrogen energy 32 (2007) 3154- 3158.
3. [3] L.W. Wang, R.Z. Wang, R.G. Oliveira A review on adsorption working pairs for refrigeration. Renewable and Sustainable Energy Reviews 13 (2009) 518–534
4. [4] J.V.Veselovskaya, R.E. Critoph, R.N. Thorpe, S. Metcalf, M.M. Tokarev, Yu.I. Aristov Novel ammonia Sorbents ‘‘porous matrix modified by active salt” for adsorptive heat transformation: 3.Testing of BaCl2/vermiculite composite in a lab-scale adsorption chiller. Applied Thermal Engineering 30
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 3, March-2014 450
ISSN 2229-5518
(2010) 1188-1192 6. [6] N.K. Bansal, J.Blumenberg, H.J. Kavasch, T. Roettinger.
5. [5] Y. Zhong, R.E. Critoph, RN. Thorpe, Z. Tamainot-Telto, Yu.I. Aristov Isothermal sorption Characteristics of the BaC12- NH3 pair in a vermiculite host matrix. Applied Thermal
Engineering 27 (2007) 2455-2462
Performance testing and evaluation of solid absorption solar cooling unit.Solar Energy 61(1997) 127-140.
IJSER © 20 14