International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 332
ISSN 2229-5518
Oladipo Mary Adelaide, Semire Banjo*, Osunlana Ronke Ruth, and Onawumi O.O. Esther
Department of Pure and Applied Chemistry, Ladoke Akintola University of Technology, Oyo
State, Ogbomoso, Nigeria.
*Corresponding e-mail: bsemire@lautech.edu.ng
The copper (II) complexes of 4,4,4-trifluoro-1- (3-pyridyl)-1,3-butanedione(ftbdH), 1-(2- furyl)-1,3-butanedione (fbdH), 1-phenyl-1,3-butanedione (bzacH) and their adducts with 1,10- phenanthroline have been synthesized and characterized accordingly by elemental analysis, solubility, infra-red and electronic spectroscopic methods. The complexes and adducts were insoluble in water, slightly soluble in ethanol but soluble in dimethylformamide, chloroform, tetrachloromethane and acetone. Infrared spectral of the complex and adducts revealed that lower frequency shifts of varying magnitudes were observed in the carbonyl (C=O) and (C=C) aromatic stretching vibrations when compared with that of their ligand values. Electronic spectral data also indicated the geometries of the complex and adducts and transitions in terms of
π-π*. The experimental vibration frequencies are compared with those obtained from semi-
empirical (PM3) level of calculations based on the proposed structures.
Di-ketones are carbonyl compounds with two ketonic groups as their functional group. However, separation of these two ketonic groups by a methyl group gives rise to β-diketones. β- diketones and their metal complexes are among the most widely studied coordination compounds
due to their wide application in the industries as catalyst (Schwieger, 2009; Xing bang, 2009;
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 333
ISSN 2229-5518
Ferreira, 2002; Poncelet, 2005; Lassahn, 2005; Campelo, 2006) and also as precursors for chemical vapor deposition (Zhang , 2006; Nable et al, 2003; Banger et al, 2001; Dela Rosa et al,
2003). The transition metal complexes of β-diketones have been the subject of many different studies ranging from synthetic, kinetic, antimicrobial and structural topics to catalysis and many others (Plessis, et al, 1998; Campelo et al, 2006; Lassahn et al, 2005; Xingbang et al, 2009; Schwieger et al, 2009). Theoretical methods have been used to study copper complexes of β- diketones in order to gain insight into their structural and electronic interactions as related to their practical uses (Dela Rosa et al, 2003). β-diketonates are a class of high functional compounds with outstanding optical, electric and magnetic properties and the negative ion may act as an excellent chelating agent (Wang, et al., 2006; Halim et al., 2005). The isolation of various substituted β-diketones complexs have been reported (Woods et al, 1994; Odunola et al,
2003).
Thermal transfer printing materials containing metal β-diketonates exhibit good whiteness and image stability (Miura et al, (1993)). It has been found that toners containing metal complexes of β -diketones are stable, controllable and capable of producing clear colour images even at high temperatures and high humidity without producing copier stain (Hiroshi and Katsuhiko, 1987). Studies on the effect of substituent on the spectra properties of metal β- diketonates have been reported (Nakamoto et al, 1959; Nakamoto et al, 1962; Patel and Woods,
1990; Woods and Patel, 1994). However, there is dearth of information on 2-substituted-1- phenyl-1, 3-butanedione copper (II) complexes and their 2,2′-bipyridine and 1,10-phenanthroline adducts. Therefore, in this work we concentrate on the synthesis, spectroscopic and theoretical studies of the Copper (II) substituted-1-phenyl-1, 3-butanedione , 4,4,4-trifluoro-1- (3-pyridyl)-
1, 3 butanedione , 1-(2-furyl)-1,3-butanedione and their adducts with 1,10-phenanthroline.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 334
ISSN 2229-5518
The complexes and its adducts are modeled based on the spectroscopic interpretations using Spartan program (Spartan 06) implemented on an Intel Pentium M 2.0GHz computer. The optimization and frequency calculation of the Cu (II) complex and its adducts were performed using semi-empirical method (PM3), since PM3 has been successfully used either alone or with other theorertical methods for structural analysis of Cu(II) complexes (Johnson et al, 2000; Bernabe et al, 2001; Seguel et al, 2005; Adeoye et al, 2010; Sequel et, al, 2010 and Adekunle et al, 2011; Oladipo et al, 2012). The Mulliken charges and highest molecular orbitals (HOMO) of adducts and the metal complexes were reported.
The results of the elemental analysis, colour, percentage yield, melting points/decomposition temperatures and room temperature magnetic moments (Ueff) of the complexes are given in Table 1.
All the compounds were obtained as various shades of green and blue except for Cu
(fbd)2 .2H2 O and Cu(Ftbd)2 phen3 that were yellow.
The results of elemental analysis of the complexes and adducts studied are shown in Table 1.The observed results agree well with the expected ones suggesting that the complexes were 1: 2 metal: ligand as expected for divalent complexes while the addition compounds were obtained as
1: 1 adducts.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 335
ISSN 2229-5518
![]()
![]()
![]()
CF 3
O
N
O O
![]()
![]()
1 2 H 1 2
![]()
![]()
H 2+ O O Cu
![]()
![]()
![]() H
H

3 O O 4
![]()
![]()
![]()
O O 2 H H ![]()
Cu 2+ O
![]()
O
![]()
![]()
![]() H
H
3O O 4
![]()
1O H H ![]() 2+ O
2+ O
![]()
O Cu
![]()
![]()
![]() H H O O
H H O O
3
N CF 3
O
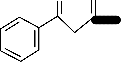
A = [Cu(Fbd)2]2+.2H2O
B = [Cu(Bzac)2]2+.2H2O
C = [Cu(Ftbd)2]2+.2H2O
H C
3 H C
3

O
![]()
N O
![]()
![]()
1 Cu 2+ O
O
![]()
![]()
![]()
N 1O
N O
2 3 O 2
O
2+
Cu
N O 2
2 3 O
H C
3
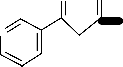
D = [Cu(Fbd)2.phen]2+
H 3 C
E = [Cu(Bzac)2.phen]2+
F 3 C

N O
![]()
![]()
![]()
N 1O
2+
Cu
N O 2 N
2 3 O
F 3 C
F = [Cu(Tf db)2.phen]2+
Figure 1: The proposed structures of the complexes and adducts with numbering of atoms.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 336
ISSN 2229-5518
%Cu | %C | %H | µeff B.M | ||||||||
Compound | Molecula r mass | Color | % Yield | Melting point | Ex | Ob | Ex | Ob | Ex | Ob | |
Cu(ftbd)2 .2H2 O | 541.93 | Green | 83 | >220 | 11.73 | 11.56 | 35.16 | 35.24 | 2.23 | 2.19 | 2.06 |
Cu(fbd)2.2H2 O | 1046.5 | Yellow | 39 | 18-220 | 6.07 | 6.00 | 59.68 | 59.62 | 3.08 | 3.04 | 1.90 |
Cu(Bzac)2 .2H2 O | 421.94 | Blue | 92 | 162-164 | 15.06 | 15.07 | 56.93 | 56.90 | 5.26 | 5.25 | 2.03 |
Cu(fbd)2 .phen | 566.12 | Green | 27 | 220 | 11.22 | 12.51 | 67.89 | 68.02 | 4.99 | 5.23 | 1.89 |
Cu(Bzac)2 .phen | 566.115 | Green | 21 | 208-210 | 11.22 | 12.51 | 67.89 | 68.10 | 4.99 | 4.70 | 2.19 |
Cu(Ftbd)2 phen3 | 1046.522 | Yellow | 39 | 218-220 | 6.07 | 6.00 | 59.68 | 59.62 | 3.08 | 3.04 | 1.99 |
The complexes and adducts had varying degree of solubilities in both coordinating and non-coordinating solvents. They were all insoluble in water, slightly soluble in ethanol but very soluble in dimethylformamide, chloroform, tetrachloromethane and acetone.
The observed and theoretical vibrational frequencies of the complexes and adducts and their tentative assignments are presented in Table 2. The spectra of β –diketonates have been studied and assignment made for the different bands in the spectra of the compounds overlap in the absorption frequencies of most vibrational modes of these ligands due to electron delocalization in the chelate ring results in appreciable coupling of the different vibrational modes (Nakamoto, k, 1970). The most important bands in the β –diketone ligands are the C=O and C=C and the CH3 rocking vibration because they are metal-sensitive. In 1-phenyl-1,3- butanedione, the assymmetric stretching vibration of C=O + C=C appear as three (1590cm-1,
1550cm-1 and 1515cm-1). Conversely, in 1-(2-furyl)-1,3-butanedione, these bands appear around
1725cm-1 and 1610cm-1 while in 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione, they were at
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 337
ISSN 2229-5518
1655cm-1 and 1620cm-1, the CH3 group being replaced by CF3 . The position of the ʋasc=o + ʋasc=c stretching vibrations which is higher in fbdH than in ftbdH could probably be attributed to a more electronegative oxygen atom in the furyl ring than sulphur and nitrogen in the thienyl ring which had affected the extend of the mesomeric interactions occuring between these five membered heterocyclic rings and possibly the corresponding metal chelate ring. Flourine substitution shifts the ʋasc=o + ʋasc=c band to higher frequencies, a phenomenon commonly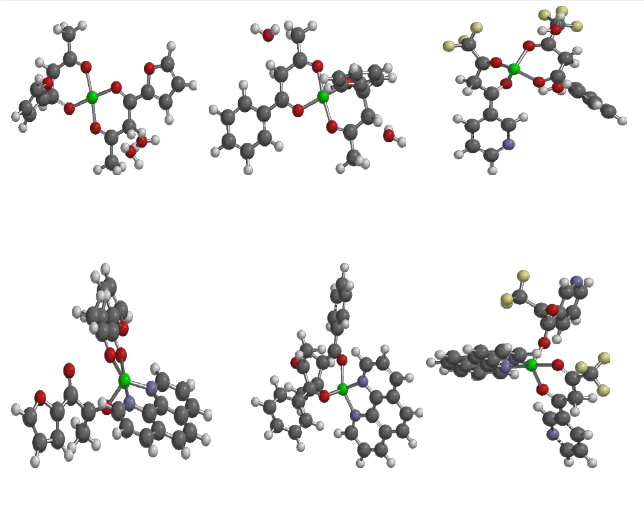
found with fluorinated β –diketones.
(A) [Cu (fbd)2. 2H2O] (B) [Cu (Bzac)2. 2H2O] (C) [Cu (ftbd)2. 2H2O]
(D) [Cu (fbd)2. phen] (E) [Cu (Bzac)2.phen] (F) [Cu (Tfbd)2. phen]
Figure 2: Optimized structures of the complexes and adducts at PM3 level of semi-empirical method
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 338
ISSN 2229-5518
The main stretching modes in the infrared spectra of the complexes and adducts were shifted to shorter wavelength of absorption in the complex relative to the parent ligand. The ʋc=o
(cm-1) modes of copper complexes observed at lower frequency shifts relative to the parent
ligands could be an indication of stronger Cu-O bonds in the chelate ring due to increased electron delocalization. The infrared spectra of the complexes and adducts showed that lower frequency shifts were observed in the carbonyl stretching frequencies on complexation. This was evident in all the complexes and adducts. For instance, ʋc=o vibrational frequency for FbdH
was found at 1725cm-1 but in the complex and adduct, it was found at 1558cm-1 and 1600 cm-1
respectively. Theoretically, these were calculated to be 1772, 1543 and 1603 cm-1 for FbdH, complex and adduct respectively. Infrared studies on diketones have shown that electron releasing substituent give rise to low ʋc=o (cm-1). This indicates that the β-diketonate ligands adopt a chelating coordination mode (Harding, et. al. 2008). Although calculated vibrational frequencies at PM3 were higher than the experimental values, the scaled vibrational frequencies agreed very well with experimental values. The ʋc=o stretching frequencies of adducts were relatively lower than that of the parent ligands and complexes, this was consistent with the theoretical results.
Compound | Experimental ʋC=O (cm1) | Computational (unscaled) ʋC=O (cm1) | Computational (scaled with 0.895) ʋC=O (cm1) |
FtbdH | 1655, 1620 | 2012, 1970 | 1801, 1763 |
FbdH | 1725, 1610. | 1980, 1963 | 1772, 1757 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 339
ISSN 2229-5518
BzacH | 1590(m), 1550(s) | 1979, 1963 | 1771, 1757 |
Cu(ftbd)2 .2H2 O | 1580(s), 1545(s) | 1916, 1849 | 1714, 1655, |
Cu(fbd)2.2H2 O | 1558(w), 1525(w) | 1724, 1678 | 1543, 1501 |
Cu(Bzac)2 .2H2 O | 1600s , 1568s, 1534s , 1504s | 1738, 1724, 1647, 1623 | 1555, 1543, 1474, 1452 |
Cu(Bzac)2 .phen | 1595w , 1570s , 1510s | 1853, 1805, 1703 | 1604, 1562, 1474 |
Cu(ftbd)2 phen3 | 1575(s), 1540(s) | 1860, 1780, 1763 | 1665, 1593, 1578 |
Cu(fbd)2 .phen | 1600w, 1576w , 1560w | 1852, 1815, 1612 | 1603, 1570, 1439 |
The electronic spectra of the diketones, thier copper complexes and adducts both in methanol and chloroform are in Table 3. The assignment of the bands have been made based on related compunds.(Frackler,1966, Singh and Sahai, 1969, Fleming and Thorton, 1975). The π-π* intraligand transitions within the phenyl, furyl and thienyl rings were observed within the 36000-
41000cm-1 region. This could be due to semi-empirical calculations on the π – electron system
.(Brailer et al,1973). The π3 -π4 * transitions appeared in the 28000-34000cm-1 region. However, the d-d bands of the compounds were found 14000-18000cm-1 region. On complexation, bathochromic shifts were observed in the π3 -π4 * transitions of the complexes in methanol and chloroform except Cu(Fbd)2 with hypsochromic in methanol. The electronic spectra of copper(II) complexes are difficult to interprete due to different ordering of the energy levels [Fackler et al,1963]. The six coordinate copper (II) ion with a 2Eg ground state in an octahedral field is subject to Jahn-Teller distortion. The visible absorption bands of copper(II) β-diketonates are more intense and located at lower in methanol (a coordinating solvent) than in chloroform (a
non-coordinating solvent) [Golchoubian and Khoshiar, 2012]. The effect of ligand and solvent
variation in these spectra has been interpreted in some cases as indicating square pyramidal
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 340
ISSN 2229-5518
structure. A shift to lower frequency of the d-d transitions in these complexes in methanol could be in indication of a change from four coordinate, square planar stereochemistry to five coordinate geometry, a shift to higher frequency indicates a change from five coordinate to six coordinate while no spectral shift indicates six coordinate copper(II) species [Addison et al,
1981].
In the Copper(II) complexes studied, a plausible four coordinate geometry is proposed based on the spectra shifts of the d-d transitions. There are shifts to lower frequencies of these transitions. The π3 -π4 * transitions of the complexes in methanol were accompanied by bathochromic shifts on adduct formation while the d-d transitions of these complexes were accompanied by hypsochromic shifts on adduct formation. In the adduct Cu(Ftbd)2 phen3 , there was a shift of the π3 -π4 * transitions to lower frequency because of the presence of fluorine. The hypsochromic shifts probably indicate weaker delocalization as a result of stronger copper-base interraction. There was a high frequency shift of the d-d transitions of the adducts on changing solvent from chloroform to methanol. This is consistent with the coordinating ability of methanol resulting in six-coordinate copper(II) ions and could be an indication of a change from five coordinate, possibly square pyramidal, stereochemistry to six-coordinate geometry in methanol. Consequently, a probable five coordinate geometry is proposed for the adducts.
Compound | d-d × 103 cm-1 transition | π 3 -π4 * × 103 cm-1 | π-π*x103 (ph, Fu & py) | |||
Compound | Methanol | Chloroform | Methanol | Chloroform | methanol | Chloroform |
FbdH | 28.28(502) | 28.61(12505) | 36.80(4271) | 36.93(21883) | ||
Ftbdh | 28.01(678s) | 29.26(13604) | 35.42(5244) | 36.20(20484) | ||
BzacH | 32.68(1070) | 33.33(17440) | 40.65(397) | 40.80(33363) | ||
Cu(Ftbd)2 .2H2 O | 14.62(21) | 14.95(40) | 29.76(39149) | 29.64(37680) | 36.90(13940) | 35.71(13200) |
Cu(Fbd)2.2H2 O | 15.15(27.43) | IN | 30.21(31686) | IN | 36.23(16643) | IN |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 341
ISSN 2229-5518
Cu(Bzac)2 .2H2 O | 14.90(38) | 15.42(49) 17.91(49) | 31.45(39382) | 31.56(38000) | 39.84(25598) | - |
Cu(Fbd)2 .Phen | 16.26(73) | 14.16(56) | 29.59(49097) | 30.67(43532) | 33.78(102790) | 33.67(17925) |
Cu(Ftbd)2 .Phen3 | 15.61(46) | 15.88(60) | 28.70(38568) | 28.68(37589) | 37.45(100438) | 35.71(142685) |
Cu(Bzac)2 .Phen | 16.50(106) | 14.22(53) | 30.36(17000) | 30.58(17273) | 40.49(27902) |
NB: Values in bracket indicates the absorptivity of the band.
The room temperature magnetic moments for the copper (II) complexes and adducts were between 1.90-2.10 B.M which is within the range expected for magnetically dilute copper(II) ion(Earnshaw and Greenwood, 1984). An increase in the moment was observed in the adducts as compared with the parent complexes. This probably indicates reduced electron density in the chelate ring.
The schematic structures and optimized structures of both complexes and adducts are shown is Figures 1 and 2 respectively. The complexes and adducts were modeled based on the data obtained from infrared spectra, electronic spectra and elemental analysis of complex and adducts. The geometric parameters calculated at PM3 level of the semi-empirical method are listed in Table 4. The calculated bond distances of Cu-O1, Cu-O2, Cu-O3, and Cu-O4 are 1.849,
1.822, 1.847 and 1.842Å for complex A respectively. These bond distances were 1.851, 1.826,
1.852 and 1.845Å for Complex B and 1.925, 1.874, 1.910 and 1.955Å for complex C
respectively. The average four Cu-O bond distances from the metal ion-ligand complexation for
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 342
ISSN 2229-5518
A, B and C are 1.840, 1.844 and 1.916Å respectively, therefore Cu-O is stronger in complex A
as compared to either A or C; the order Cu-O bond in the complexes are A > B > C.
Table4: Geometry parameters for the complexes using PM3 level of semi-empirical method.
Bond Distance (Å) | A [Cu(fbd)2 .2H2 O] | B [Cu(Bzac)2 .2H2 O] | C [Cu(ftbd)2 .2H2 O] |
Cu1-O1 | 1.849 | 1.851 | 1.925 |
Cu1-O2 | 1.822 | 1.826 | 1.874 |
Cu1-O3 | 1.847 | 1.852 | 1.910 |
Cu1-O4 | 1.842 | 1.845 | 1.955 |
Average Cu-O | 1.840 | 1.844 | 1.916 |
BOND ANGLES (0) | |||
O1-Cu1-O2 | 99.30 | 97.43 | 127.97 |
O1-Cu1-O3 | 83.50 | 83.81 | 107.00 |
O1-Cu1-O4 | 173.27 | 171.47 | 110.70 |
O2-Cu1-O3 | 176.30 | 176.66 | 104.67 |
O2-Cu1-O4 | 73.98 | 75.93 | 105.33 |
O3-Cu1-O4 | 103.20 | 102.94 | 96.61 |
In adducts, the Cu-O1, Cu-O2 and Cu-O3 bond distances are 1.967, 1.932 and 1.924Å for D, these bonds are calculated to be 1.959, 1.928 and 1.930Å for E respectively. In adduct F; these bonds are 1.960, 1.931 and 1.936Å respectively (Table 5). The average Cu-O bond distances for adducts D, E and F are 1.940, 1.939 and 1.942Å respectively, therefore Cu-O is relatively shortened in the complexes than that of adducts, thus Cu-O bonds are stronger in the complexes than in adducts. This is consistent with the both experimental and calculated infrared spectra which show lower frequency shifts indicating stronger Cu-O bonds in the complexes. The Cu-N1 (Cu-N2) bond distances for adducts D, E and F are 1.924 (1.915), 1.922 (1.918) and
1.919Å (1.916Å) respectively. Comparing the values of Cu-O and Cu-N bond distances, Cu-N
bonds are stronger than Cu-O bonds (longer bond distances) in adducts, therefore adducts
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 343
ISSN 2229-5518
formation weakened the Cu-O bonds which are consistent with experimental infrared spectra obtained.
HOMO
LUMO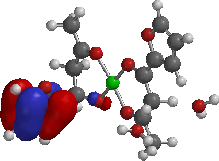 (A)
(A) 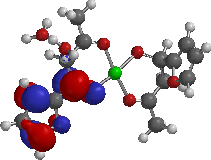
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 344
ISSN 2229-5518
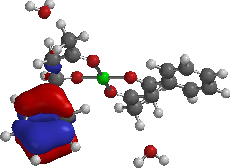
(B) (C)
(C)
Figure 3: Frontier Molecular orbitals: HOMO and LUMO of the complexes.
The O1-Cu-O2, O1-Cu-O4, O2-Cu-O4 and O3-Cu-O4 bond angles are 99.30, 173.27,
73.98 and 103.20º respectively for complexes A. These bond angles are 97.43, 171.47, 75.93 and
102.94º for complex B and 127.97, 110.70, 105.33 and 96.61º respectively for complex C. From the Table 4, it could be suggested that complexes A and B adopted square planar geometries and complex C tetrahedral geometry.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 345
ISSN 2229-5518
In adducts, O1-Cu-O2, O1-Cu-O3 and O2-Cu-O3 bond angles are 115.24, 88.39 and
90.01º for adduct D; 115.21, 88.80 and 89.41º for adduct E and 98.39, 88.67 and 88.90º for adduct F respectively. The N1-Cu-N2, O1-Cu-N1, O2-Cu-N1, O2-Cu-N2 and O3-Cu-N2 bond angles are 90.79, 103.93, 140.72, 87.36 and 174.44º for D; 90.78, 104.43, 141.32, 87.01 and
174.62º for E and 94.77, 94.90, 175.44, 89.61 and 138.15º for adduct F respectively. The trigonal bipyramidal geometry was proposed for adducts.
Table 5. Geometry parameters for adducts using PM3 level of semi-empirical method.
BOND DISTANCE (Å) | D [Cu(fbd)2 .phen] | E [Cu(Bzac)2 .phen] | F Cu(Ftbd)2 .Phen |
Cu1-O1 | 1.967 | 1.959 | 1.960 |
Cu1-O2 | 1.932 | 1.928 | 1.931 |
Cu1-O3 | 1.922 | 1.930 | 1.936 |
Cu1-N1 | 1.924 | 1.922 | 1.919 |
Cu1-N2 Ave. Cu-O | 1.915 1.940 | 1.918 1.939 | 1.916 1.942 |
BOND ANGLES (0) | |||
O1-Cu1-O2 | 115.24 | 115.21 | 98.39 |
O1-Cu1-O3 | 88.39 | 88.80 | 88.67 |
O2-Cu1-O3 | 90.01 | 89.41 | 88.90 |
O1-Cu1-N1 | 103.93 | 104.43 | 94.90 |
O1-Cu1-N2 | 97.17 | 96.34 | 123.38 |
O2-Cu1-N1 | 140.72 | 141.32 | 175.44 |
O2-Cu1-N2 | 87.36 | 87.01 | 89.61 |
O3-Cu1-N1 | 88.14 | 89.50 | 87.78 |
O3-Cu1-N2 | 174.44 | 174.62 | 138.15 |
N1-Cu1-N2 | 90.79 | 90.78 | 90.77 |
IJSER © 2013 http://www.ijser.org
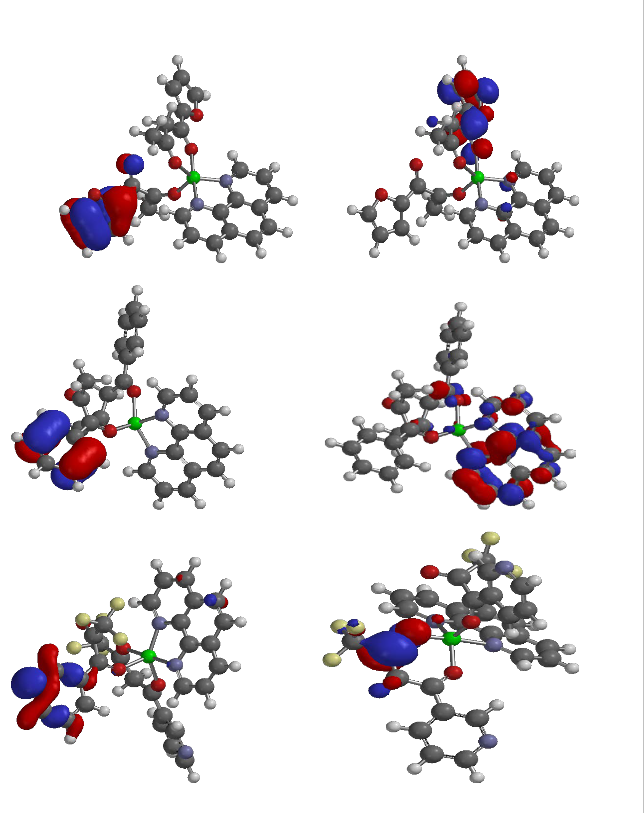
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 346
(D)
(E)
(F)
Figure 4: Frontier Molecular orbitals: HOMO and LUMO of adducts.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 347
ISSN 2229-5518
The contour plots of HOMO and LUMO orbitals of the complexes and adducts computed at the PM3 level of semi-empirical are displayed in Figures 3 and 4 respectively. The HOMO of complex A was localized on the furan ring of one of the ligand, while the LUMO is principally localized on one furan and C=O of 1-furyl-1,3-butanedione. For complex B, the HOMO is localized on one of the benzene rings of the ligand and LUMO is localized mainly on the one of the benzene rings and C=O of 1-phenyl-1,3-butanedione of the ligand. For complex C, both HOMO and LUMO are localized on pyridinyl-1,3-butanedione.
For adducts D, E and F (i.e. [Cu (Bzac)2 .phen], [Cu (fbd)2 .phen] and [Cu(Tfbd)2 .phen]), the HOMOs are localized on the furan ring of 1-furyl-1,3-butanedione, benzene ring of 1-phenyl-
1,3-butanedione and pyridine ring of the pyridinyl-1,3-butanedione in D, E and F respectively (i.e. the HOMO map of these adducts is on the ligand that used one of its C=O in bonding to cupper ion). The LUMO map of adducts is mainly localized on the phenanthroline. The HOMO-LUMO band gap of the complexes suggested that the complexes and adducts could have good stability.
Table 6: Mulliken charges of the complexes.
Atom | A [Cu(fbd)2 .2H2 O] | B [Cu(Bzac)2 .2H2 O] | C [Cu(ftbd)2 .2H2 O] |
Cu1 | -0.149 | -0.169 | -0.381 |
O1 | -0.011 | -0.025 | -0.143 |
O2 | -0.132 | -0.116 | -0.236 |
O3 | -0.049 | -0.052 | 0.008 |
O4 | 0.050 | 0.058 | 0.018 |
Atom | D [Cu(fbd)2 .phen] | E [Cu(Bzac)2 .phen] | (F) [Cu(Tfbd) 2 .phen] |
Cu1 | -0.353 | -0.363 | -0.370 |
O1 | -0.141 | -0.170 | -0.178 |
O2 | -0.112 | -0.110 | -0.047 |
O3 | -0.150 | -0.152 | -0.144 |
N1 | 0.504 | 0.507 | 0.502 |
N2 | 0.500 | 0.506 | 0.493 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 348
ISSN 2229-5518
The Mulliken charges on copper atom in the complexes A, B and C are -0.149, -0.169 and - 0.381respectively, this is an indication that electrons were donated into d-orbital of the copper atom from the ligands. The charges on copper ion in complex C is higher than that of A and B which implied that more electrons are available in the d-orbital of copper ion of complex C. This might be due to the three atoms of chlorine which behaved as electron donating specie rather than electron abstracting specie coupled with higher electron donating ability of pyridine over furan and benzene. The same argument could be used to explain a Mulliken charge on copper ions of adducts D, E and F. The Mullikan charges of D, E and F are -353, -363 and -370e respectively (Table 6). Evidence of electrons transfer during complexation could be seen in the Mulliken charges on coordinated carbonyl oxygen atoms; the charges on O1 (O2) in complexes A, B and C are -0.011 (-0.132), -0.025 (-0.116) and -0.143 (-0.236e) respectively as compared to
-0.231 (-0.286), -0.216 (-0.284) and -0.223 (-0.196e) in FbdH, BzacH and FtbdH ligands respectively. The Mullikan charges on O4 are all positive; 0.050, 0.058 and 0.018e in complexes A, B and C respectively.
The Mulliken charges on O1 (O2) for adducts D, E and F are -0.141 (-0.112), -0.170 (-
0.110) and -0.178 (-0.047) respectively which are reduced as compared to the ligands. This supports experimental results that more lone pair of electrons is donated to Copper from the ligands. The Mulliken charges on nitrogen atoms are all positive depicting that the lone pair of electrons on nitrogen atoms have been donated to the central Copper ion in the formation of adducts. The nitrogen atoms N1 (N2) have Mulliken charges 0.504 (0.500), 0.507 (0.506) and
0.502e (0.493e) in complex D, E and F respectively.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 349
ISSN 2229-5518
Copper (II) complex of 4,4,4-trifluoro-1-(3-pyridyl)-1,3-butanedione (FtbdH), (1-(2- furyl)-1,3-butanedione (FbdH) and 1-phenyl-1,3-butanedione, ( BzacH) were synthesized and characterized by elemental analysis, infrared and electronic spectra . The metal analysis agreed with the expected theoretical values. There was a hypsochromic shift in the carbonyl stretch vibrational band on ligand-metal ion complexation and also in adduct formation. This was in agreement with the theoretical results. The proposed structures of the complexes and adducts based on the electronic spectra results were modeled by quantum chemical software package. The geometries parameters were calculated at PM3 level of semi empirical methods, the HOMO, LUMO and Mulliken charges of the complexes and adduct were also reported.
Adekunle F.A., Oladipo M.A and Semire B. (2011) Synthesis, spectroscopic characterization and theoretical studies of Cu(II)2 H2 O(ClO4 )2 with some bridging ligands, Science Focus, 16(1), 60-68.
Adeoye I. O. Odunola O. A., Oladipo M. A and Semire B. (2010) Quantum chemical study on molecular and electronic structures of methyl and methoxyl substituted Cu(II) and Ni(II) benzoic acid hydrazides ions. E-journal of chemistry. 7(2), 517-525
Anthony W. Addison, Hugo M.J. Hendriks, Jan. Reedijk, Lawrence, K. Thompson(1981)
Copper complexes of the “tripod” ligand tris(2-benzimidazolylmethyl) amine: five- and six-coordinate copper(I) derivatives. Inorg. Chem, 20(1) pp 103-110
Bao-Qing Ma, Song Gao, Zhe-Ming Wang, Chun-Sheng Liao, Chun-Hua Yan, and Guang-Xian Xu, (1999) Synthesis and structure of bis(dibenzoylmethane) copper(II). Journal of chemical crystallography, Journal of chemical crystallography, 29(7), 793-796
Bernabé L., Rivas B.L., Gloria V. Seguel G.V and Kurt E. Geckeler K.E. Poly(styrene-alt- maleic acid)–metal complexes with divalent metal ions. synthesis, characterization, and
physical properties. J. App. Poly. Sci. 86(6), 1310-1015.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 350
ISSN 2229-5518
CampeloJ.M., Jaraba M., Luna D., Luque R., Marinas J.M and Romero A.A. (2006).Structural and catalytic properties of amorphous mesoporous AlPO4 materials prepared in the presence of 2,4-pentanedione and 2,5-hexanedione as aluminium chelating agents. Studies in surface science and catalysis, 162, 315-322.
DelaRose, M.J., Banger, K.K., Higashiya, S., Ngo, S.C. (2003). Structural investigations of copper(II) complexes containing fluorine-substituted beta-diketone ligands. Journal of Fluorine Chemistry, 123, 109-117.
Halim, M.A, Nessa, S.A, Rahman, A.K.L, Chowdhury, D.A and Salam, M.A (2005). Studies on diazocoupling products of dioxomolybdenum(VI) chelates of beta- diketones, Journal of applied sciences 5(6), 1027-1031
Hamid Golchoubian and Farida Khoshiar (2012). Substistuent effect in the ᵧ-position of
acetylacetonate on the solvatochiomic property of bis(acetylacetonate) copper (II)
complexes. Chemistry Journal, vol02 issue 01, pp1-8
Harding, P., Harding, D.J., Phonsri, W., Saithong, S., Phetmung, H., (2008). Synthesis and electrochemical studies of octahedral Nickel beta- diketonate complexes, Inorganica Chimica Acta
John P. Fackler, Jr., F.A.Cotton and D.W. Barnum, (1963) Electronic spectra of β-Diketone complexes III. α-substituted β-diketone Complexes of copper (II). Inorg. Chem. 2(1), pp
97-101.
Johnson J.S and Evans D.A. (2000) Chiral Bis(oxazoline) Copper(II) Complexes: Versatile Catalysts for Enantioselective Cycloaddition, Aldol, Michael, and Carbonyl Ene Reactions. Acc. Chem. Res. 33,325-335
Lassahn P.G., Lozanm V., Timco G.A., Christian P., Janiak C. and Winpenny R.E.P. (2005).Homo and Heterometallic carboxylate cage complexes as precatalysts for olefin polymerization – activity enhancement through inert metals’. Journal of Molecular catalysis, 222(1), 260-267.
Nakamoto K., Udovich C. and Takemoto J.,(1970); Metal isotope effect on metal ligand vibrations IV; Metal complexes of Acetylacetone, Vol.183, pg.459.
Ogden D. and Selbin J., (1968) ultraviolet spectra studies of beta-ketoenolate complexes of oxovanadium(IV), Journal of Inorganic and Nuclear chemistry 30, 1227-1236.
Oluwatola O., Joseph W., (2011) Synthesis and Physicochemical Studies of Nickel(II) Complexes ofVarious 2-Alkyl-1-phenyl-1,3-butanediones and Their 2,2′-Bipyridine and
1,10-Phenanthroline Adduct. International Journal of Chemistry,3 (1), 24-35.
Oladipo M. A., Semire B, Adeagbo A. I and Johnson J. A. (2012) Synthesis, spectroscopic characterization and theoretical studies of Copper (II) 1, 3-diphenylpropane-1, 3-dione complex and its 1, 10-phenanthroline, 2, 2-bipyridine adducts. International Journal of
Pure and Applied Science and Technology (in press)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 10, October-2013 351
ISSN 2229-5518
Patel K.S and Woods J.A.O (1990).Synthesis and properties of nickel(II) complexes of various
3-alkyl-2,4-pentanediones and their adducts with 2,2-bipyridine and 1,10-phenanthroline.
Synthesis and reactivity in Inorganic and metal organic chemistry, 20(4), 409-424.
Patel K.S and Faniran J.A (2001) Physicochemical studies of metal beta-diketonate III, Journal of inorganic and Nuclear chemistry (Dublin) 39, 1143-1147.
Plessis, W.C, Vosloo, T.G, and Swarts, J.C (1998). Beta- diketones containing a terrocenyl group; synthesis, structural aspects, pKa values, group electronegativities and complexation with rhodium(I), Journal of chemical society, Dalton Trans 2507-2514
Schwieger S., Herzog R., Wagner C., Steinborn D. (2009).Platina-beta-diketones as catalysts for hydrosilyation and their reactivity towards hydrosilanes. Journal of Organometallic Chemistry, 694(22), 3548-3558.
Seguel G. V., Rivas B.I and Novas C. (2005) polymeric ligand-metal acetate interactions.
Spectroscopic study and semi-empirical calculations. J. Chil. Chem. Soc. 50, (1), 401-
406.
Seguel G. V., Rivas B.I, Paredes C. (2010) Study of the interactions between copper (II) acetate monohydrate and orotic acid and orotate ligands. J. Chil. Chem. Soc. 55, (3), 355-358
Tayyari, S.F, Rahemi, H., Nekoei, A.R., Zahedi-Tabrizi, M., Wang, Y.A (2006). Vibrational assignment and structure of dibenzoylmethane, a density functional theoretical study. Spectrochimica Acta Part A 66, 394-404.
Turgambaeva, A.E, Bykov, A.F and Igumenov, I.K (1995). Mass spectrometric study of Copper (II) beta-diketonates vapour thermolysis mechanism and kinetics. Journal De physique IV, C5-221.
Woods J.A.O. and Patel K.S. (1994). Nickel(II) complexes of some 3-substituted-2,4- pentanediones and their adducts with 2,2’-bipyridine and 1,10-phenanthroline. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 24(9), 1557-1571.
Wang, Y.H, Yu, O.S, Zou, J.W, Lu, Y.X, Xu, H.Y (2006). Theoretical study on enol- ketotautomerism of alpha- fluorine- beta- diketones, Chinese journal of structural chemistry 25(3), 363-367
Xingbang H., Jianyong M., Yong S., Hang C., Haoran L. (2009).AcetylacetoneFe catalyst modified by imidazole ionic compound and its application in aerobic oxidation of beta- isophrone. Catalysis communications, 10(14), 25.
IJSER © 2013 http://www.ijser.org