International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1131
ISSN 2229-5518
Synthesis and characterization of nano-sized copper oxide by X-ray diffraction & scanning electron microscopy
R. P. Ugwekar1, G. P. Lakhawat 2*
1Department of Chemical Engineering, Laxminarayan Institute of Technology, Nagpur-440019
2*Department of Chemical Engineering, Priyadarshini Institute of Engineering & Technology, Nagpur-440 019
Abstract
The synthesis and characterization of nanosized copper oxide have been done in the present investigation. The prepared nanoparticles are characterized using X-ray diffraction and scanning Electron Microscope. XRD pattern result shows the presence of most intense peak curresponding to (4800) crystallographic orientation of the spinel phase of CuO nanoparticles. The mean size of nanoparticles was determined from X ray diffraction pattern by using the Scherrer approximation. The particle size was calculated to be 40.086.
* Corresponding author
—————————— ——————————
1 INTRODUCTION
The interest in nanomaterials has increased in recent year because of their unique physical and chemical properties.Copper oxide based material have been widely investgated due to their potential application in many fields.The oxides of transition metals are an important class of semiconductor. Several studies have been done for its characterstics. Among the oxides of transition metal, copper oxide nanoparticles are of special interest because of their efficiency as nanofluids in heat transfer application.
In this paper we have synthesized CuO nanoparticles by chemical precipitation methods which give size of nanoparticle
40.086 nm . The synthesized nanoparticles were characterized by XRD and SEM.
2 Materials and methods
Experimental Part
Chemical precipitation is probably the most common method to prepare copper oxide nanoparticle. In this
method copper nitrate is used as a precursor and sodium bicarbonate as precipitating agent.
Synthesis of Copper oxide nanoparticles
The nanoparticles of Copper oxide was synthesized by chemical precipitation method in which copper
nitrate (0.1 M) and sodium bicarbonate solution (0.1 M) were prepared in distilled water[8]. The sodium
bicarbonate solution was added drop wise under constant speed of stirring to copper nitrate with reaction
allowed to proceed for 2 hr until complete addition of sodium bicarbonate with PH being kept 6-8. After the
completion of reaction by testing the complete precipitation , the precipitate was allowed to settle overnight
and then filtered off and precipitate was washed several times with distilled water until free from excess
bicarbonate that may present. A pale blue color precipitate was observed and the supernatant solution was
then discarded carefully. After washing , the paste cake shape were dried at 80 oc for 3 hr and then calcined at
350oc and 450oc for 3 hr as well.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1132
ISSN 2229-5518
3 Result and Discussion
Nanoparticle characterization is necessary to establish understanding and control of nanoparticle synthesis. Characterization is done by using variety of different technique such as x ray diffraction and scanning electron microscope.
(a) Characterization of CuO Nanomaterial using X-ray Diffraction (XRD)
X-ray powder diffraction (XRD) is an analytical technique primarily used for phase identification of a crystalline material and can provide information on unit cell dimensions [1].
In X-ray diffraction method, the incident monochromatic radiation strikes the finely powdered contained in capillary tube. The photographic film is wrapped around the inside of cylindrical chamber concentric with the sample. The rays are diffracted from individual crystal which happened to be oriented with plane making Bragg angle θ with the beam [9].
In the powder method, the intensity of the reflected beam can also be recorded in a diffractometer which uses a counter in place of the film to measure intensities. The counter moves along the periphery of the cylinder and records the reflected intensities against 2θ.Peaks in the diffractometer recording as shown in following figure correspond to position where the Bragg condition is satisfied by some crystallographic plane [6].
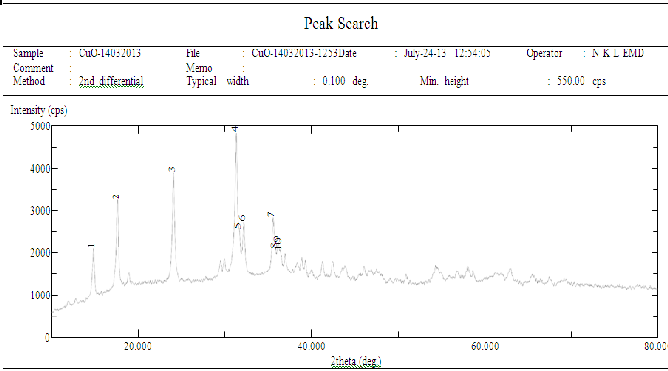
Figure 1. The tracing from diffractometer(XRD Pattern of CuO)
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1133
ISSN 2229-5518
Table 1: Peaks of Sample
Peak No. | 2 Theata(deg) | d(Ao) | FWHM(deg) |
1 | 14.800 | 5.9806 | 0.165 |
2 | 17.560 | 5.0464 | 0.235 |
3 | 24.02 | 3.7018 | 0.188 |
4 | 31.28 | 2.8572 | 0.235 |
5 | 31.64 | 2.8255 | 0.141 |
6 | 32.16 | 2.7810 | 0.188 |
7 | 35.52 | 2.5253 | 0.259 |
8 | 35.820 | 2.5048 | 0.071 |
9 | 36.14 | 2.4833 | 0.212 |
10 | 38.34 | 2.4701 | 0.141 |
Particle size calculation from X-ray diffraction:
From this study and by considering the peak at degree , average particle size has been estimated by using Debye-Scherrer formula [2]
2θ=31.28
Θ=15.64, Cos Θ=0.9629 λ =0.15406 nm
β =FWHM =0.235
β =Full width at half maximum
D= 0.9 λ/ β Cos Θ D=40.086 nm
(b) Scanning Electron Microscope of CuO Nanopowder:
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1134
ISSN 2229-5518
This is an extremely useful technique for surface investigation. The surface of nanoparticles to be examined is scanned with an electron beam and the reflected beam and reflected beam of electron is collected , then displayed at same scanning rate on the CRT [8 ].The image on the CRT represents the surface feature of the nanoparticles. In this microscopy, the surface of the nanoparticles must be electrically conductive that can be achieved by coating the surface with a very thin layer of metallic material.
Following figure shows that SEM image of the synthesized CuO nanoparticles heated at 80oc.
Figure 2 shows that general morphologies of synthesized CuO nanosphere were observed with large
number of CuO nanosphere agglomerates with a uniform size.
Figure 3 shows that the powders are composed of non-agglomerated random shape particles which
tends to built and aggregate to form a flower shape structure. The formation of soft agglomerates
with irregular morphology constituted the quite fine particles can also be seen.
The most important finding results is obtaining a sphere shape nanoparticle that can open a new
horizon in using it in nanocatalyst preparation and industry that use today a sphere shape catalyst
with larger particle size [7].
Figure 4 shows individual sphere –like nanostructure which demonstrate that the CuO
nanostructure with sphere like shape are composed of many interconnected sheet ball like crystallites
structure.

Fig. 2:- SEM image of synthesized CuO nanoparticle
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1135
ISSN 2229-5518
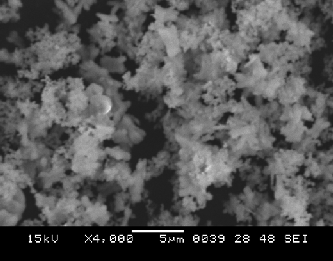
Fig. 3:- SEM image of non-agglomerated random shape CuO particles
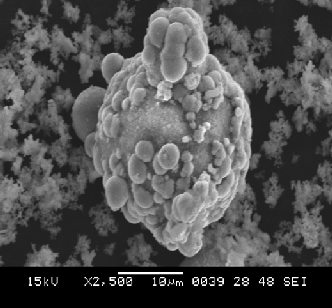
Fig. 4:- SEM image of individual CuO nanoparticle
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 2, February-2014 1136
ISSN 2229-5518
4 CONCLUSION
In the present study, average crystallite size of synthesized CuO was estimated through XRD analysis by calculation of FWHM values. This size was found to be 40.086 nm.
The present results along with known physical properties of nanocrystalline CuO offer a new dimension for further study.
REFERENCES
[1] Lanje AS, Sharma SJ, Pode RB. Synthesis and optical characterization of copper oxide nanoparticles. Advances Applied Science
Research, 2010, 1 (2):36-40.
[2] Arunachalam Dinesh Karthik and Kannappan Geetha “Synthesis of Copper Precursor, Copper and its oxide Nanoparticles by
Green Chemical Reduction Method and its Antimicrobial Activity” Journal of Applied Pharmaceutical Science ISSN 2231-3354 Vol.
3 (05), pp. 016-021, May, 2013 .
[3] D.K. Singh , D.K.Pandey & R.R.Yadav “A study of nanosized zinc oxide and its nanofluid” P RAMANA journal of physics
@ Indian Academy of science , vol 78 , No.5 , may- 2012 , p.p 759-766.
[4]E.Pradeep , Jaya Sudhan “Synthesis of nanofluids by a novel one pot methods for heat transfer application”Indian Journal of
Science & Technology , Vol.4 ,2011 , 417-421.
[5] Harikrishna Vishwanadula Emmanuel C.Nsofor , “Studies on Forced Convection Nanofluid Flow in Circular Conduits” ,ARPN Journal of Engg. and Applied Sciences Vol-7 No.3 ,2012, 371-376.
[6] Ho Chang , Min-Kun Liu “Fabrication and Process analysis of anatase type TiO2 nanofluid by an arc spray nanofluid synthesis
systeme” , Journal of Crystal Growth , 2007 ,244-252
[7] Javier A. Lopez , Ferney Gonzalez & Flavio A. Bonilla “Synthesis and characterization of Fe 3 O 4 Magnetic nanofluid” Revista
Latinoamericana de metalurgia y materials 2010 , 30(1) : 60-66 .
[8] Karim H. Hasan , Tahseen H.Mubarak Al-Bayati & Zena M. Ali Abbas “study and characterization of copper oxide nanoparticles prepared by chemical method using X-ray diffraction and scanning Electron Microscope “ American Journal of Scientific Research ISSN 2301-2005 Issue 77 october , 2012 , p.p 49-53
[9] Rakesh Dogra , Advances in Material Science , seventh edition , published by S.K.Kataria & Sons , 2008
[10] Sang-wook park, Byoung-sik choi , Seong-soo Kim , “Mass Transfer of Carbon dioxide in aqueous colloidal silica solution containin N-methyldiethanolamine” , Korean J.Chem Engg , 2008, 819-824.
[11] S.Sh.Hosseini , Adam N.M “Effect of temperature Increasing on Nanofluid Structure” , Australian Journal of Basic and
Applied Science , 2011 , 979-984.
[12] Veeranna Sridhara and Lakshmi Narayan Satapathy , “ Al2O3-based nanofluids:a review” , Nanoscale Research Letters ,
2011,1-10.
[13]Wei Yu and Huaqing Xie , “A Review on Nanofluids: Preparation , stability mechanism and Application” Environmental
Engineering , Shanghai , China.
IJSER © 2014 http://www.ijser.org



