International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014 662
ISSN 2229-5518
Fawzy A. Attaby and Ahmed A. M. Elreedy
Abstract: Reaction of diethyl 4,4'(1,4-phenylene)bis(3-amino-5-acetyl-6-methylthieno[2,3-b]pyridine-2-carboxylate (1) with hydrazine hydrate afforded the 4,4’-benzene-1,4-diylbis(5-acetyl-3-amino-6-methylthieno[2,3-b]pyridine-2-carbohydrazide) (3). The structure of 2 was inferred through independent synthetic reaction of diethyl 2,2’-{1,4-phenylenebis[3-cyano-5-acetyl-6-methylpyridine-4,2- diyl)thio]}diacetate 2 under the same applied reaction conditions. On the other hand, reaction of 3 with formic acid, acetic anhydride, triethylorthoformate, acetic acid, ethyl acetoacetate, diethylmalonate, phenylisothiocyante and acetylacetone aiming to build up pyrimidine, pyrazole or oxadiazole on the ring system of 3. Structures of all newly synthesized heterocyclic compounds in the
present study were confirmed by considering the data of IR, 1H NMR, mass spectra as well as that of elemental analyses.
Index Terms: bis-thienopyridine-2-carboxylate; bis-thienopyridine-2-carbohydrazide; bis-pyridothienopyrimidin-4(3H)-one); bis- pyridothienopyrimidin-3(4H)-yl)imidoformate; bis-pyrazolothienopyridin-3-one)
![]()
1 INTRODUCTION
The biological importance of both bis-compounds [1-5] and 2-thioxopyridine-3-carbonitriles [6-11] as well as a conjunction to our previous work [12-18] stimulated our interest to synthesize several derivatives of these ring systems that are required for several chemical transformations and for our medicinal chemistry programs.
2 RESULTS AND DISCUSSION
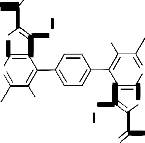
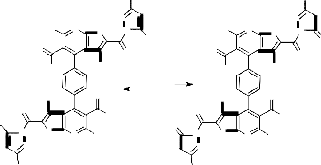
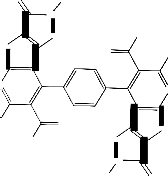
An unequivocal support for the structure of compound 1 [19] came from the series of reactions concerning the presence of the ester group at 2- position of the thienyl moiety. Thus, it has been found that diethyl 4,4'(1,4-phenylene)bis(3-amino-5- acetyl-6-methylthieno[2,3-b]pyridine-2-carboxylate 1 reacted with hydrazine hydrate in ethanol (20 mL) under reflux for 10 hours to give a reaction product
3 that corresponding to the loss of two molecules of ethanol. The IR spectrum (cm-1) of 3 showed the absorption bands of the NH2 and NH groups at
---------------------------------------
Fawzy A. Attaby: Professor of Organic Chemistry,
Cairo University, Faculty of Science, Chemistry Department, Giza 12613, Egypt; E- mail: fattaby@hotmail.com; Ahmed A. M. El- Reedy: Basic-Applied Sci. Dept., Faculty of Oral and Dental Medicine, Nahda University, Beni-Suef, Egypt
3454, 3322 and 3219. Its mass spectrum gave the peaks at m/z = 603, 0.5% which corresponding to the parent peak of the molecular ion [M+H]+ in addition to other peaks which gave a further confirmation of 3 structure (cf. Exp. Part and Scheme 1). Based on the above data, in addition to that of elemental analysis, this compound could be formulated as 4,4’-benzene-1,4-diylbis(5-acetyl-3- amino-6-meth-ylthieno[2,3-b]pyridine-2-carbohyd- razide) 3. An authentic sample of compound 3 was obtained by the reaction of compound 2 with hydrazine hydrate under reflux for 10 hours. It important to report here that compound 3 obtained by the two pathways was indentical in all physical and chemical properties (cf. Exp. Part). The isolation of compound 3 with their adjacent NH2 and CONHNH2 groups stimulated our interest to utilize it as a versatile starting material for the synthesis of several heterocyclic derivatives. This goal was achieved via its reactions with a variety of activated reagents aiming to build up the pyrimidine nucleus on the thienopyridine skeleton. Thus, it has been found that compound 3 reacted with formic acid under reflux to give the corresponding 4,4’-benzene-1,4-diylbis(8-acetyl-3- amino-7-methylpyrido[3’,2’:4,5]thieno[3,2-d]pyrimi- din-4-(3H)-one) 4. The reaction most probably proceeded through the formylation of NH2 group at the thiophene ring to give the non-isolable intermediate [I] which converted to the enol form [II] followed by elimination of two molecules of
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014 663
ISSN 2229-5518
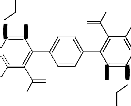
COOEt
S CN
CH3
O
CH3
NH2NH2.H2O
![]()
N N
Reflux
H3C
O H3C
2

COOEt
NC S COOEt
H2N
NH O
S NH2
CH3
O
CH3
NH2NH2.H2O
NH2
S
CH3
O
CH3
N N N N
Reflux
H3C
O H3C
H2N
S
COOEt
H3C
O H3C
H2N
O
S
![]()
NH2
NH
![]()
1 3
HCOOH -H2O Reflux
![]()
O NHNH2
S N
OH H3C
![]()
O
CH3
![]()
O NHNH2
CHO S NH
H3C
![]()
O
CH3
![]()
N N N N
H3C
H3C
O N S HO
O NHNH2
H3C
H3C
O
NH
OHC
S
O NHNH2
II I

NH2

O N
-H2O
N
S H3C
O
CH3
N N
H3C
O S N
H3C
N O
Scheme 1
H2N
4
water to afford the final isolable reaction product 4 (cf. Scheme 1). The IR spectrum (cm-1) of 3 showed the absorption bands of NH2 group at 3452, 3326 and acetyl CO group at 1736 beside ring CO group at 1678. The mass spectrum of 4 gave m/z = 623 (0.2%, [M+H]+) which corresponded to the
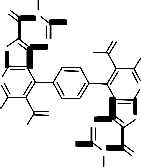
molecular formula C30 H22 N8 O4 S2 of the assigned structure (cf. Exp. Part). In a further investigation, compound 3 reacted with acetic anhydride under reflux for 5 hours to give the corresponding 4,4’- benzene-1,4-diylbis(8-acetyl-3-amino-2,7-dimethyl- pyrido[3’,2’:4,5]thieno[3,2-d]pyrimidin-4(3H)-one)
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014 664
ISSN 2229-5518
5. The reaction most probably proceeded by the acetylation of NH2 group at the thiophene ring through the intermediate [I] followed by enolization and elimination of two molecules of water from the intermediate [II] to afford the final isolable reaction product 5 (cf. Scheme 2). The IR spectrum (cm-1) of this reaction product showed
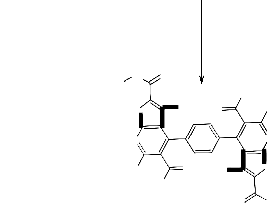
the absorption bands of NH2 group at 3454, 3326 and CO groups at 1748 and 1697 and its mass spectrum gave m/z = 651 (7.7%, [M+H]+) which corresponded to the molecular formula C32 H26 N8 O4 S2 of the assigned structure (cf. Exp. Part and Scheme 2).![]()
NHNH2
O
COCH3
N H
![]()
CH3
![]()
(CH3CO)2O
3
Reflux 5hrs.
-CH3COOH
S CH3CO
N N
H3C
COCH3
H N CH3CO
![]()
I
S
O NHNH2
NH2
O N
N
CH3
O
![]()
![]()
H2N
O NH
![]()
OH
CH3
N
S H3C
CH3
-2H2O
S CH3CO
CH3
![]()
N N N N
H3C
H3C
O
H3C
S H3C N
N O
COCH3
H3C
N S
![]()
O OH HN
5 H2N
II NH2
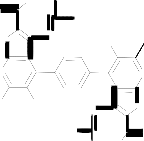
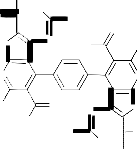
Moreover, compound 3 reacted with triethylorthoformate under reflux for 5 hours to afford the corresponding 6. The IR spectrum (cm-1) of 6 neither showed absorption bands of NH nor NH2 groups while absorption bands at 1691 cm-1 was detected. Thus we concluded that two molecules of triethylortho-formate reacted with 3 to afford the non-isolable intermediate [I] which in turn, reacted with another two molecules of triethylorthoformate to afford the final isolable 6 via the non-isolable product [II] (cf. Scheme 3). Moreover, its mass spectrum gave the parent peak at m/z = 735 which corresponding to the molecular weight of the assigned structure in
addition to other peaks that gave further confirmation of the structure 6 (cf. Exp. Part). As a further continuation of the interest of exploring the synthetic potential of compound 3 it was thus of value to investigate its reaction with β-dicarbonyl compounds such as ethyl aceto-acetate, acetylacetone and diethylmalonate 7a-c to afford a new heterocyclic derivatives. Thus, it has been found that compound 3 reacted with ethyl acetoacetate 7a in glacial acetic acid under reflux for 5 hours to give 5,5'-diacetyl-3,3'-diamino-2-(3- methyl-5-hydroxy-1H-pyrazole-1-carbonyl)-6,6'-dimeth- yl-4,4'-benzene-1,4-diylbisthieno[2,3-b]pyridine 8a.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014 665
ISSN 2229-5518

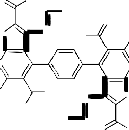
HC(OEt)3
3 Reflux
-4 C2H5OH
NH2
![]()
O N
N S
H3C
![]()
O
CH3
NHNH2
![]()
O EtO
S N
H
O H3C
![]()
CH3
N N N N
H C O
![]()
-2 C2H5OH
3 S N
H3C
H3C
O S N
H3C
N O H2N
H O OEt
H2NHN
II I
![]()
![]()
OEt
-4 C2H5OH
N O N
O N
S H3C
CH3

HC(OEt)3 N N
H3C
O S N
H3C
N O N
6
EtO
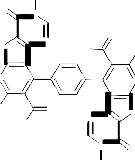
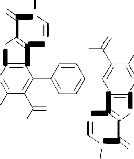
The reaction most probably proceeded via the nucleophilic addition of hydrazidic NH2 of 3 on the carbonyl group of 7a to give the non-isolable intermediate Ia . The formation of Ia followed by elimination of two water molecules of to afford the non-isolable intermediates IIa which in turn, underwent cyclization via the elimination of two ethanol molecules to afford the final isolable reaction product 8a rather than 8`a whose structure was elucidated by considering the data of IR, mass spectral data as well as that of elemental analysis (cf. Exp. Part, Equation 1 and Scheme 3). Similarly, compound 3 reacted with acetylacetone 7b under reflux for 5 hours in glacial acetic acid to afford the corresponding 5,5'-diacetyl-3,3'-diamino-2-(3,5-di- methyl-1H-pyrazole-1-carbonyl)-6,6'-dimethyl-4,4'-
benzene-1,4-diylbisthieno[2,3-b]pyridine 8b. The IR spectrum (cm-1) of 8b showed the absorption bands of NH2 group at 3454, 3382 and CO group at
1684 and its mass spectrum gave m/z = 731 (0.5%, [M+H]+) which corresponded to the molecular weight of the molecular formula C38 H34 N8 O4 S2 of the assigned structure. Several peaks at m/z = 636 (100%, 731- dimethylpyrazole ring), 608 = (0.1%,
731- one O=C – di methyl pyrazole ring), 511 =
(4.6%, 731- one O=C - two dimethylpyrazole ring – two H), and 483 = (0.2%, 731- two dimeth- ylpyrazole – two C=O – two H) gave further confirmation of the structure 8b. The reaction most probably proceeded via the addition followed by elimination of two molecules of water through the non-isolable intermediate Ib and IIb followed by
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014 666
ISSN 2229-5518
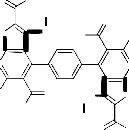
cyclization through the elimination of another two molecules of water to afford the final isolable reaction product 8b (cf. Scheme 4 and Equation 1). In a similar manner, compound 3 reacted with diethylmalonate 7c under reflux to afford the corresponding 4,4'-benzene-1,4-diylbis-5-acetyl-3- amino-2-(1H-pyrazol-3,5-dione-1-carbonyl)-6-methyl- thieno[2,3-b]pyridine 8c whose structure was confirmed via the elemental analysis and spectral data (cf. Exp. Part and Scheme 4). 7,7'-Diacetyl-6,6'- dimethyl-8,8'-benzene-1,4-diylbis(1,2-dihydro-3H-
R -CO-CH -R
pyrazolo[3',4':4,5]thieno[2,3-b]pyridin-3-one) 9 obtained via the reaction of compound 3 with glacial acetic acid under reflux for 5 hours. The IR (cm-1) of compound 9 showed the absorption bands of acetyl CO, ring CO as well as NH groups and its mass spectrum gave the parent peak at m/z = 684, (0.3%, [M+H]+) which corresponding to the molecular weight of the assigned structure (cf. Exp. Part and Scheme 5). 4,4'-Benzene-1,4-diylbis-5- acetyl-3-amino-6-methyl-2-(N-phenyl-1,3,4-oxadiazol-
R1-CO-CH2-R2
1 2 2
3
(7a-c)
(7a-c)
b
-2 H2O
a, c
-2 EtOH (in a)
-4 EtOH (in c)
R3
H3C N O
CH3
N
S N
R4
O NH2
H2N O
R4
N S N
R3
N
8a-c
O
CH3

CH3
2-amine-5-yl)thieno[2,3-b]pyridine 10 obtained through the reaction of compound 3 with phenyl isothiocyanate under reflux in pyridine for 5 hours. The IR (cm-1) of this reaction product showed the absorption bands of NH2 and C=O functional groups as well as its mass spectrum gave the parent peak at m/z = 805 which corresponding to the molecular weight of the assigned structure 10 (cf. Exp. Part and Scheme 5). Compound 3 reacted with 2-cyano-3-(4- methoxy-phenyl)prop-2-enethioamide 11 in pyridine under reflux to give a reaction product
which formulated as 4,4'-benzene-1,4-diylbis-5- acetyl-3-amino-6-methyl-N’-(4-methoxyphenylmeth- ylidene)thieno[2,3-b]pyridine-2-carbohydrazide 13 (cf. Scheme 5). The IR spectrum (cm-1) of 13 showed the absorption bands of NH2 groups at
3460, 3317 and C=O at 1666 cm-1. Its mass spectrum gave m/z = 838 which corresponded to the molecular formula C44 H38 N8 O6 S2 of the assigned structure (cf. Exp. Part). An unequivocal support for the structure of 13 was achieved via its
synthesis by another route, via using anisaldehyde
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014 667
ISSN 2229-5518
12 to give a reaction product which was found completely identical in all aspects with 13 obtained from the first route (cf. Scheme 5 and Exp. Part). Therefore, we concluded that the reaction between
3 and 2-cyano-3-(4-methoxyphenyl)prop-2-enethio-
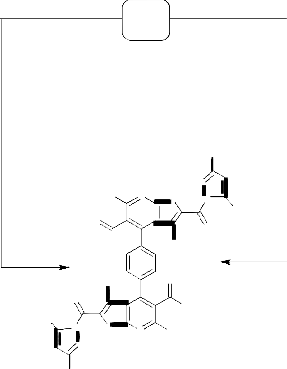
amide 11 most properly proceeded via the ylidine group exchange with the loss of one molecule of 2- cyanoethanethioamide to afford the corresponding reaction product 13.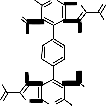
![]()
H3C N O
![]()
H3C R
OH
S
NH2
![]()
O
NH NH
![]()
CH3
HO
R
![]()
- 2 H2O
H3C N O
H3C
S
NH2
![]()
O
NH N
H3C R
H3C
H2N O
CH3
H2N O
NH NH
O S
CH3
N CH3
R N NH
O S
CH3
N CH3
![]()
[I]a,b [II]a,b
Ia, IIa Ib, IIb
R, CO2Et COCH3
Addition
R1-CO-CH2-R2
-2 H2O (in IIb)
-2 EtOH (in IIa)
3 H3C
H3C
H3C N O
CH3
N
S N
R'
O NH2
H3C N O
CH3
N
S N
O
O NH2
H2N O
R'
O
CH3
H2N O
O
O
CH3
20a, R' = OH
20b, R' = CH3
N
N
CH3
S N
7a,b
CH3
N
N
CH3
S N
6a,b
CH3
Equation 1
3 EXPERIMENTAL
All melting points were uncorrected. I.R. (KBr discs) spectra were recorded on a Shimadzu FTIR-
8201PC Spectrophotometer. 1H-NMR spectra were recorded on a Varian Mercury 300 MHz., and a Varian Gemini 200 MHz. Spectrometers using TMS as an internal standard and CDCl3 , DMSO-d6 , and (CD3 )2 CO as solvents. Chemical shifts were expressed as δ (ppm) units. Mass spectra were recorded on Agilent LC 1200/MS Ion Trap 6320 using APCI ionization source and the spectra is enhanced using acidified water/acetonitril mobile
phase and measured in the positive mode of the ion trap (Molecular weights of most compounds are Protonated ([M+H]+).
Synthesis of (3): A solution of 1 or 2 (1g, 0.63 mol) in hydrazine hydrate (15mL) and ethanol (20 mL) was heated under reflux for 10 hrs., the excess solvents were evaporated and the reaction mixture then cooled and the solid so formed was collected by filtration, dried, and crystallized from ethanol to give 3.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014 668
ISSN 2229-5518
CH3COOH
O NH2
N
N
S Ac O
CH3
CH3
![]()
N N
H3C
H3C
O
Ac
N
N
H2N O
9
H5C6HN
N
O N
C6H5NCS
S NH2
CH3
O
CH3
3
N N
(12)
H3C
O H3C
H2N S
MeOH4C6
N
O N
10 N H5C6HN
NH O
S
OMe-p-C6H4-CH=C(CN)CSNH2 (11)
NH2
CH3
O
CH3
N N
H3C
O H3C
S H2N
![]()
N
13 O
NH ![]()
C6H4OMe
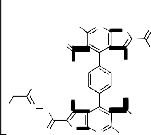
4,4'-Benzene-1,4-diylbis(5-acetyl-3-amino-6-meth- ylthieno[2,3-b]pyridi-ne-2-carbohydrazide) (3): as yellow crystals yielded by (83%); m.p>3300C; IR υ(cm-
1): 3454.1,3322.6 (NH2 ), 3219.7 (NH), 2984.1,2920.4 (aliphatic-CH), 1691.6 (Acetyl-CO), 1642.4 (Amide-
CO); MS: 603 ([M+H]+, 0.5% which corresponding to the molecular weight of the molecular formula C28 H26 N8 O4 S2 of the assigned structure) 585 (603- H2 O, 0.4%), 572 (603-NHNH2 , 42.9%), 571 (603-
NHNH2 , H, 100%), 544 (572-CO, 17.9%), 543 (571- CO, 60.2%), 513 (544-NHNH2 , 2.6%), 512 (543- NHNH2 , 0.6%), 485 (513-CO, 0.3%), 484 (512-CO,
0.8%); 1H NMR(DMSO) (δppm): 1.952(s, 6H,
2CH3 ), 2.521(s, 6H, 2COCH3 ), 4.487(br, 4H, 2NH2 ),
5.793(br, 4H, 2NH2 ), 7.606(m, 4H, ArH`S), 9.171(br,
2H, 2NH); Anal, for C28 H26 N8 O4 S2 (602) Calcd./Found(%): C(55.80/55.83%) H(4.35/4.38%)
N(18.59/18.62%) S(10.64 /10.67%).
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014 669
ISSN 2229-5518
Synthesis of 4, 5, 6, and 9 (General method): A solution of 3 (0.6g, 1mmole) and each of15ml of (Formic acid, Acetic anhydride, Triethylortho- formate, and Acetic acid), was heated under reflux for 5 hrs. The excess solvent was evaporated and cooled. The solid was collected by filtration, dried, and crystallized from the ethanol to give 4, 5, 6, and 9 respectively.
4,4'-Benzene-1,4-diylbis(8-acetyl-3-amino-7-meth- ylpyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4(3H)- one) 4: as yellow crystals yielded by (93%); m.p>3300C; IR υ(cm-1): 3452.7, 3326.4 (NH2 ),
2923.3,2852.4 (aliphatic-CH), 1736.1 (CO), 1678.6 (CO); MS: 623 ([M+H]+, 0.2% which corresponding to the molecular weight of the molecular formula
C30 H22 N8 O4 S2 of the assigned structure), 607 (623- NH2 , 7.2%), 606 (623-OH, 9.6%), 605 (623-H2 O,
29.2%), 590 (605-NH, 7.4%), 589 (605-NH2 , 21.6%),
587 (605-H2 O, 100%), 563 (590-HCN, 1.6%); 1H NMR (DMSO) (δppm): 2.172 (s, 6H, 2CH3 ), 2.504 (s, 6H, 2COCH3 ), 6.082 (br, 4H, 2NH2 ), 7.471 (m,
4H, ArH`S); Anal for C30 H22 N8 O4 S2 (622) Calcd./Found(%): C(57.87/57.90%) H(3.56/3.59%)
N(18.00/18.03%) S(10.30/10.33%).
4,4'-Benzene-1,4-diylbis(8-acetyl-3-amino-2,7-di-
methylpyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4- (3H)-one) 5: as white crystals yielded by (78%); m.p>3300C; IR υ(cm-1): 3454.4,3326.3 (NH2 ), 1748.3 (CO), 1697.8 (CO); MS: 651 ([M+H]+, 7.7% which corresponding to the molecular weight of the molecular formula C32 H26 O4 N8 S2 of the assigned structure), 636 (651-CH3 , 4.5%), 635 (651-NH2 ,
30.4%), 634 (651-OH, 54.5%), 633 (651-H2 O, 100%),
620 (636-NH2 , 34.9%), 619 (633-CH3 , 23.6%), 618 (634-NH2 , 17.1%), 615 (634-H2 O, 77.3%), 603 (618- CH3 , 4.9%),; Anal, for C32 H26 O4 N8 S2 (650) Calcd./Found(%): C(59.06/59.09%) H(4.03/4.06%) N(17.22/17.25%) S(9.86/9.89%).
4,4'-Benzene-1,4-diylbis(ethyl(8-acetyl-7-methyl-
4-oxopyrido[3',2':4,5]thieno[3,2-d]pyrimidin-3(4-
H)-yl)imidoformate) 6: as yellow crystals yielded by (83.7%); m.p > 3300C; IR υ(cm-1): 2921.1,2852.4 (aliphatic-CH), 1691.6 (Acetyl-CO); MS: 735 ([M+H]+, 0.1% which corresponding to the molecular weight of the molecular formula C36 H28 N8 O6 S2 of the assigned structure), 690 (735- OEt, 1%), 677 (735-2Et, 0.6%), 663 (690-HCN, 2.3%),
661 (690-Et, 4.5%), 643 (735-2EtOH, 0.3%), 621 (677-![]()
2CO, 100%), 606 (621-NH, 0.8%);1H NMR (DMSO) (δppm): 1.031 (t, 6H, 2CH2CH3 ), 2.018 (s, 6H, 2CH-![]()
3 ), 2.163 (s, 2H, 2CH), 2.504 (s, 6H, 2COCH3 ), 4.322 (q, 4H, 2CH2 CH3 ), 7.563 (m, 4H, ArH`S); Anal, for C36 H28 N8 O6 S2 (734) Calcd./Found(%): C(58.84/58.87%) H(4.12/4.15%) N(15.25/15.28%) S(8.73/ 8.76%).
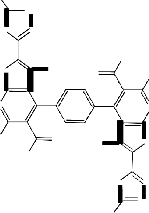
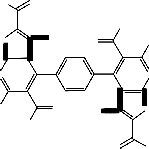
7,7'-Diacetyl-6,6'-dimethyl-8,8'-benzene-1,4-diylbis- (1,2-dihydro-3H-pyrazolo[3',4':4,5]thieno[2,3-b]pyrid-
in-3-one) 9: as yellow crystals yielded by (82%); m.p
>3300C; IR υ(cm-1): 2997.5,2884.2 (aliphatic-CH),
1695.6 (Acetyl-CO), 1673.3 (pyrazol-CO); MS: 684
([M+H]+, 0.3% which corresponding to the molecular weight of the molecular formula C32 H26 N8 O6 S2 of the assigned structure), 641 (684- COCH3 , 7.2%), 640 (684-CH3 CHO, 15.3%), 638 (640-H2, 100%), 626 (641-NH, 18.4%), 596 (640- CH3 CHO, 3.6%), 595 (638-CH3 CO, 2.4%), 594 (638- CH3 CHO, 4.6%), 582 (626-CH3 CO, 3.8%), 580 (596- NH2, 3.5%), 563 (595-2NH2 , 1.6%); Anal, for C32 H26 N8 O6 S2 (682.7) Calcd./Found(%): C(56.30/56.33%) H(3.84/3.87%) N(16.41/16.44%) S(9.39/9.42%).
Synthesis of 8a-c (General method): A solution of each of 3 (0.6g 1mmole) and 15ml of Ethyl 3- oxobutanoate, acetylacetone and diethylmalonate, (0.26g, 0.2g, 0.32g 2 mmole) was heated in 20ml of acetic acid under reflux for 5 hrs. The excess
solvent was evaporated and cooled. The solid was collected by filtration, dried, and crystallized from the ethanol to give 8a-c respectively.
5,5'-Diacetyl-3,3'-diamino-2-(3-methyl-5-hydroxy-
1H-pyrazole-1-carbonyl)-6,6'-dimethyl-4,4'-benz- ene-1,4-di-ylbisthieno[2,3-b]pyridine (8a): as yellow crystals yielded by (67%); m.p>3300C; IR υ(cm-1): 3482.1,3392.4 (NH2 ), 1684.8 (CO); MS: 733 (M-H, 0.1% which corresponding to the molecular weight of the molecular formula C36 H30 N8 O6 S2 of the assigned structure), 716 (733-OH, 10.6%), 715 (733-H2 O, 25.5%), 697 (715-H2 O, 14.1%), 680 (697- OH, 10.8%), 636 (733-C4 H5 ON2 , 28.4%), 635 (715- C4 H4 N2 , 8.8%), 619 (636-OH, 100%), 607 (635-CO,
30.1%), 600 (680-C4 H4 N2 , 9.9%); Anal for
C36 H30 N8 O6 S2 (734) Calcd./Found(%): C(58.84-
/58.87) H(4.12/4.15) N(15.25/15.28) S(8.73/8.76).
5,5'-Diacetyl-3,3'-diamino-2-(3,5-dimethyl-1H-
pyrazole-1-carbonyl)-6,6'-dimethyl-4,4'-benzene-
1,4-diylbis-thieno[2,3-b]pyridine (8b): as Brown
crystals yielded by (76%); m.p = 2600C; IR υ(cm-1):
3454.3,3382.2 (NH2 ), 1684.5 (CO); MS: 731 ([M+H]+, 0.5% which corresponding to the
molecular weight of the molecular formula C38 H34 N8 O4 S2 of the assigned structure), 637 (731- C5 H6 N2 , 46.8%), 636 (731-C5 H7 N2 , 100%), 635 (731-C5 H8 N2 , 34.5%), 621 (637-NH2 , 0.8%), 608 (636-CO, 0.1%), 607 (635-CO, 2.1%), 512 (608- C5 H8 N2 , 2.8%), 511 (607-C5 H8 N2 , 4.6%), 484 (512- CO, 0.7%), 483 (511-CO, 0.2%); Anal, for C38 H34 N8 O4 S2 (730) Calcd./Found(%): C(62.45/62.48%) H(4.69/4.72%) N(15.33/15.37%) S(8.77/ 8.8%).
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014 670
ISSN 2229-5518
4,4'-Benzene-1,4-diylbis-5-acetyl-3-amino-2-(1H- pyrazol-3,5-dione-1-car-bonyl)-6-methylthieno[2,3- b]pyridine (8c): as Brown crystals yielded by (76%); m.p>3300C; IR υ(cm-1): 3479.4,3389.6 (NH2 ), 1691.7 (CO); MS: 739 ([M+H]+, 0.2% which corresponding to the molecular weight of the molecular formula C34 H26 N8 O8 S2 of the assigned structure), 723 (739- NH2 , 1.3%), 721 (739-H2 O, 11.5), 703 (721-H2 O,
3.1%), 697 (739-CH2 CO, 100%), 696 (739- CH3 CO,
13%), 679 (697-H2 O, 17.3%), 661 (679-H2 O, 8.7%),
640 (739-C3 H3 O2 N2 , 8.7%), 639 (721-C3 H2 ON2 ,
4%), 622 (721-C3 H3 O2 N2 , 2.1%); Anal, for
C34 H26 N8 O8 S2 (738) Calcd./Found(%): C(55.28/55.31%) H(3.55/3.58%) N(15.17/15.2%)
S(8.68 /8.71%).
Synthesis of 10: A solution of each of 3 (0.6g,
1mmole) and phenyl isothiocyanate, (0.27g,
2mmol) in pyridine (15 mL) was heated under
reflux for 5 hrs, cooled, poured onto ice-cold water,
and neutralized with drops acetic acid the solid was collected by filtration, dried, and crystallized from the ethanol to give 10.
4,4'-Benzene-1,4-diylbis-5-acetyl-3-amino-6-meth-
yl-2-(N-phenyl-1,3,4-oxadiazol-2-amine-5-yl)thieno- [2,3-b]-pyridine (10): as Brown crystals yielded by (81%); m.p = 2250C; IR υ(cm-1): 3464.4,3378.2 (NH2 ),
3055.5 (aromatic-CH), 1688.2 (Acetyl-CO); MS: 805 (M, 0.1% which corresponding to the molecular weight of the molecular formula C42 H32 N10 O4 S2 of
the assigned structure), 790 (805-NH, 37.2%), 789 (805-NH2 , 100%), 773 (789-NH2 , 4%), 771 (789- H2 O, 34%), 755 (773-H2 O, 5.9%), 754 (771-OH,
5.8%), 635 (789-2Ph, 7.2%); Anal, for C42 H32 N10 O4 S2 (804) Calcd./Found(%): C(62.67/62.7%) H(4.01/4.04%) N(17.40/17.43%) O(7.95/7.98%) S(7.97/ 8.00%).
Synthesis of 13 (Method A): A solution of each of
3 (0.6g, 1mmole) and 2-cyano-3-(4-methoxy
phenyl)prop-2-enethioamide 11 (0.44g, 2mmol) in
pyridine (15 mL) was heated under reflux for 5 hrs, cooled, poured onto ice-cold water, and neutralized with drops acetic acid the solid was collected by filtration, dried, and crystallized from the ethanol to give 13.
Method B: A solution of each of 3 (0.6g, 1mmole)
and 4-methoxybenzaldehyde 12 (0.27g, 2mmol) in pyridine (15 mL) was heated under reflux for 5 hrs, cooled, poured onto ice-cold water, and neutralized with drops of acetic acid the solid was collected by filtration, dried, and crystallized from the ethanol to give 13.
4,4'-Benzene-1,4-diylbis-5-acetyl-3-amino-6- methyl-N’-(4-
methoxyphenylmethylidene)thieno[2,3-
b]pyridine-2-carbohydrazide (13): as yellow crystals
yielded by (77%); m.p >3300C; IR υ(cm-1):
3460.4,3317.5 (NH2 ), 1666.5 (Amide-CO); MS: 838 (M, 0.4% which corresponding to the molecular
weight of the molecular formula C44 H38 N8 O6 S2 of the assigned structure), 731 (838-PhOCH3 , 100%),
713 (731-H2 O, 36.4%), 705 (731-CN, 7.2%), 688 (731- CH3 CO, 9.8%), 662 (705-NH,CO, 17.1%), 624 (731- PhOCH3 , 5.6%), 606 (624-H2 O, 15.3%); Anal, for
C44 H38 N8 O6 S2 (838) Calcd./Found(%): C(62.99/63.02%) H(4.57/4.60%) N(13.36/13.39%)
O(11.44/11.47%) S(7.64/ 7.67%).
4 References
[1] D. Braun and R. Langendorf, J. Prakt. Chem.,
341, 128, 1999.
[2] A. A. Abbas, M. A. A. Elneairy and Y. N.
Mabkhot, J. Chem. Res., 124, 2001.
[3] A. R. Bhat, F. Ather and A. Azam, Eur. J. Med. Chem., 44, 426, 2009.
[4] A. M. Abdel-Fattah and M. M. Elsayed, Curr. Org. Chem., 13, 1751, 2009.
[5] G. Galli, M. Laus, A.S. Angeloni,
Makromol.Chem., 187, 289, 1986.
[6] A. M. Shestopalov, K. G. Nikishin, A. V.
Gromova, L. A. Rodinovskaya, Russ. Chem. Bull., Int.
Ed., 2003, 52, 2203.
[7] S. Prachayasittikul, L. Treeratanapiboon, S.
Ruchirawat, V. Prachayasittikul, Excli Journal 8, 121, 2009.
[8] E. S. Al-Abdullah, Molecules, 16, 3410, 2011.
[9] Dawoud, N. T. A., J. American Sci. 9, 202, 2011.
[10] H. M. F. Madkour, M. A. L. Salem, M. L.
Marzouk, M. E. Azab and N. F. H. Mahmoud, Am.-
Eurasian J. Scientific Res., 2, 161, 2007.
[11] E. G. Paronikyan, Sh. F. Akopyan, A. S. Noravyan, L. A. Dzhagatspanyan, L. M. Nazaryan, A. G. Akopyan, Pharmaceutical Chem. J., 44, 183, 2010. [12] F. A. Attaby, A. M. Abdel-Fattah, L. M. Shaif M. M. Elsayed, Phosphorus, Sulfur, Silicon, Relat. Elem.,
185, 668, 2010.
[13] F. A. Attaby, A. M. Abdel-Fattah, L. M. Shaif, M.
M. Elsayed, Phosphorus, Sulfur, Silicon, Relat. Elem.,
185, 129, 2010.
[14] F. A. Attaby, A. H. Elghandour, M. A. Ali, Y. M.
Ibrahem, Phosphorus, Sulfur, and Silicon, 182, 133, 2007.
[15] F. A. Attaby, A. H. H. Elghandour, M. A. Ali, Y. M. Ibrahem, Phosphorus, Sulfur, and Silicon, 182, 695,
2007.
[16] F. A. Attaby, M. M. Ramla, E. M. Gouda,
Phosphorus, Sulfur, and Silicon, 182, 517, 2007.
[17] F. A. Attaby, A. M. Abdel-Fattah, L. M. Shaif, M. M. Elsayed, Current Organic Chemistry, 13, 1654, 2009. [18] F. A. Attaby, A. M. Abdel-Fattah, L. M., Shaif, M. M. Elsayed, Current Organic Chemistry, 14, 2522,
2010.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 4, April-2014
ISSN 2229-5518
671
[19] F. A. Attaby, M. M. Said, Sh. M. Abu Bakr,
Egypt. ]. Chemistry, 000, 000, 2014.