The research paper published by IJSER journal is about Synthesis, Characterization and DC conductivity of Barium Hexaferrite 1
ISSN 2229-5518
Synthesis, Characterization and DC conductivity of Barium Hexaferrite
Omprakesh. S, Ameena Parveen and P.S.Naik
×10-5 eV.
Key words: Barium hexaferrite, X-ray diffraction, FTIR, SEM, Dc conductivity.
—————————— ——————————
ERRIETS Ferrites form a very good class of electrical mate- rials because of their high resistivity and low loss behav- iour, and hence have vast technological applications over a wide range of frequencies .Ferrites are preferred in the field of electronics and telecommunication industry because of their novel electrical properties which makes them useful in radiof- requency circuits, high quality filters, rod antennas, trans- former cores, read/write heads for high digital tapes and other devices . Hence it is important to study their dielectric behav- iour at different frequencies. The dielectric properties of fer- rites are dependent on several factors, such as method of preparation, heat treatment, sintering conditions, chemical
composition, cation distribution and crystallite size [1].
Ferrites, a distinct class of magnetic materials known as fer- romagnetic have spinel structure. They consist of spontane- ously magnetized domains and show the phenomena of mag- netic saturation and hysteresis. Spinel ferrites possess proper- ties of both magnetic materials and insulators and are impor- tant in many technological applications. The interesting physi- cal and magnetic properties of spinel ferrites arise from the ability of these compounds to distribute the cations among the available tetrahedral (A) and octahedral (B) sites [2]. Spinel ferrites have gained lot of attention because of their remarka- bly high electrical and magnetic flux induction. They are con- sidered as good dielectric and are found in many technological applications. Increased application of ferrites has led to the
————————————————
![]() Author name Omprakash.S Department of Physics, Gulbarga University, Gulbarga-585106, Karnataka, India. Email: omprakash_s60@yahoo.com
Author name Omprakash.S Department of Physics, Gulbarga University, Gulbarga-585106, Karnataka, India. Email: omprakash_s60@yahoo.com
![]() Co-Author name Dr.Ameena Parveen, Assistant professor in Physics, Govt.
Co-Author name Dr.Ameena Parveen, Assistant professor in Physics, Govt.
First Grade College, Guirmithkal, Yadgir, Karnataka, India. E-mail: Aparveen981@gmail.com
![]() Author of correspondence Dr.P.S.Naik, Professor in Physics, Gulbarga Uni-
Author of correspondence Dr.P.S.Naik, Professor in Physics, Gulbarga Uni-
versity, Gulbarga- 585106, Karnataka, India. E-mail: reg_psn@rediffmail.com
hydrothermal, co-precipitation and sol-gel for the preparation of stoichiometric and chemically pure spinel ferrites [3]. Ferrites having low resistivity and low eddy current losses have been found to be the most versatile to be used for techno- logical applications as in the case of stress sensors and re- cording media. Calcium ferrite possesses an inverse spinel structure and the degree of inversion depends upon the heat treatment [4], [5]. The physical properties of the spinel ferrite developed due to distribution of cations among the tetrahe- dral A site and octahedral B sites. The dielectric properties and conductivity of the ferrites depend on preparation method, chemical composition and grain size, frequency and tempera- ture [6], [7]. DC resistivity of ferrites as a function of tempera- ture and drift mobility and activation energy was reported by different authors [8], [9], [10]. Several researchers reported the
magnetic properties of spinel ferrites as well [11], [12].Ferrites
is low mobility semiconductors. To know about the conduc- tion mechanism in ferrites thermoelectric measurement is done. The thermo e.m.f. and its sign gives appropriate infor- mation about the type of conduction in semiconductors, i.e., they are p-type or n-type. There has been considerable interest during the past 10 years in finding new materials and struc- tures for use in clear, highly efficient cooling and energy con- version systems [13] – [19].
The starting materials used were barium chloride (BaCl2.2H2O), iron chloride (FeCl3.6H2O), sodium hydroxide (NaOH) and sodium carbonate (Na2CO3) of Thomas Baker, Himedia and s. d. fine chemicals.
Barium hexaferrite were synthesized for three different Fe/Ba
molar ratios 11:1, 11:2 and 11:3. Barium and iron chloride was
development of many chemical methods which includes
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 3, March-2012 2
ISSN 2229-5518
weighed in stoichiometric amount and dissolved in distilled water. After complete dissolution of chlorides and NaOH/Na2CO3 to form respective solutions; the
NaOH/Na2CO3 base solution was added drop by drop into Fe- Ba chloride solution with constant stirring till the pH of the solution became 11. The formation of complex starts precipita- tion and the precipitate obtained at pH 11 was washed several times with distilled water in order to remove the traces of NaCl from the precipitate. The precipitate was dried and presintered at 400°C for 2 hours.
The presintered precipitate was milled into fine powder for ½
hours in pestle and mortar. Polyvinyl alcohol solution in dis- tilled water was mixed to the powder along with constant milling. PVA solution was used as binder for pelletization. The powder to PVA weight percent ratio was 20:1. PVA mixed powder was pressed into tablets by hydraulic press applying pressure of 10 tons. The pellets were sintered at 750°C, in a muffle furnace under air atmosphere for 6 hours. For tempera- ture dependent conductivity, the pellets are coated with silver paste on either side of the surfaces. The copper electrodes are placed on each of the surface to obtain better contacts.
The IR spectra of the samples are recorded on Perkin-Elmer (Model 783) IR spectrometer in KBr medium at room tempera- ture. For recording IR spectra, powders are mixed with KBr in the ratio 1:25 by weight to ensure uniform dispersion in KBr pellets. The mixed powders are pressed in a cylindrical die to obtain clean discs of approximately 1mm thickness. The per- centage transmittances for the entire sample are measured from 300 to 4000 cm−1.
The X-ray diffraction (XRD) pattern of the BaFe12O19was re-
corded at room temperature by employing an x-ray powder
diffractometer (Rigaku Miniflex) with CuKα radiation (=1.5405A0) in the 2λ(Bragg angles) range (20≤ 2θ ≤100) at a scan speed of 0.50 minute-1. Optical micrographs of the films were made through a polarizing microscope (Nikon Eclipse E
400 POL) equipped with a digital camera.
The SEM images of polyaniline cadmium oxide composites were recorded using Philips XL-30 (ESEM) scanning electron microscopy. An analysis of the DC conductivity properties of the BaFe12O19 has been carried out using Kethiely meter by two probe method.
with the standard‘d’ values given in JCPDS data (84-0757). All the obtained XRD peaks were indexed with (hkl) planes from the mentioned JCPDS file which revealed formation of pure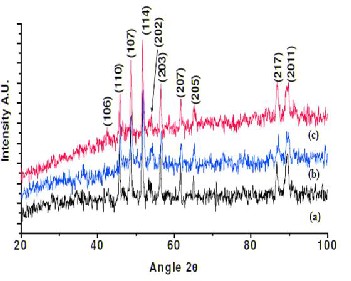
single hexagonal crystal phase.
Figure 1 (a-c) shows the XRD spectra of barium hexaferrites (Fe/Ba ratio is
11:1, 11:2 and 11:3) sintered at 7500C.
To study the doping effect of Ba+ ions on crystal structure, bar- ium hexaferrite powder of various weight percentage (Fe/Ba ratio is 11:1, 11:2 and 11:3) were sintered at 750 is shown in figure 1(a-c). For all Fe/Ba ratios, the increase in Ba+ ions con- centration in ferrites increases intensity of peaks due to in- crease in crystallinity of the material as expected. No impurity peaks were observed. Crystallite size of 11:1, 11:2 and11:3 (Fe/Ba) weight percentage barium hexaferrite calculated by Debye Scherrer’s formula is found to be 26.23 nm, 33.24 nm and 37.86 nm respectively.
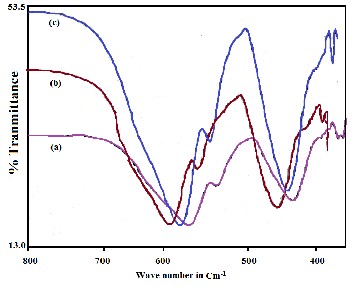
The FTIR spectrum of barium doped hexaferrite (Fe/Ba ratio is
11:1, 11:2 and 11:3) sintered at 750ºC is shown in figure 2 (a-c). All the FTIR spectra show three signature absorption peaks of hexaferrite [20] at ~ 436 cm-1, 546cm-1 and 584cm-1. The peak at
436 cm-1 indicate A2u vibration of octahedral Fe(4+)-O bonds
and other two peaks indicate E1u vibration of Fe
(3+)
O4 octahe-
The X ray diffraction patterns of barium hexaferrite powder sintered at 750ºC, is shown in figure. It revealed the formation of polycrystalline magnetoplumbite structure with preferred texture along (114). The traces of intermediate phases such as γ-Fe2O3 or BaCl2.2H2O were not observed in the X ray diffrac- tion pattern. The observed‘d’ values were in good agreement
dra [21]. In case of barium hexaferrite prepared at the ration of
11:3, the absorption at 549 cm-1 becomes higher than that of
barium hexaferrite prepared at the ration of 11:1 and 11:2. The peaks at 436cm-1 and 584cm-1 are intense showing almost 60% absorption whereas a small notch at 546 cm-1 was observed for all barium hexaferrite.
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 3, March-2012 3
ISSN 2229-5518
Figure 2 (a-c) shows the FTIR spectra of barium hexaferrites (Fe/Ba ratio is
11:1, 11:2 and 11:3) sintered at 7500C.
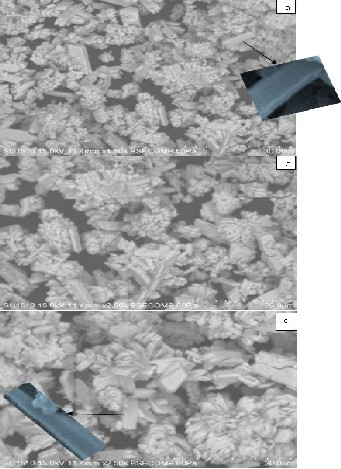
Figure 3(a-c) shows the SEM image of barium hexaferrites
(Fe/Ba ratio is 11:1, 11:2 and 11:3) sintered at 7500C.
The SEM images of 11:1, 11:2 and 11:3 barium hexaferrite bulk are shown in figure 3(a-c). The SEM image of pH11 of 11:1,
11:2 and 11:3 barium hexaferrite bulk pellets sintered at 750°C
showed various shaped grains as rectangular plates, nanorods and nanoneedles. The nanorods and nanoneedles like struc- ture was observed at some places but degree of their forma- tion was not enough to elaborate. The SEM image of barium hexaferrite (Fe/Ba ratio 11:1 & 11:2) at 7500C showed large number of vertically grown barium hexaferrite nanorods de- veloped at some places over the rectangular platelets. The rods having length ~1000nm and diameter ~80nm were developed on the surface of dense rectangular grains.
The surface morphology of 11:3 barium hexaferrite pellet re-
vealed formation of dense uniform tubular grains as depicted and comparatively more number of vertically grown long bar- ium hexaferrite nano-needles having length ~120 nm and base to tip average diameter ~50 nm developed on porous surface of plate like grains of size ~100nm. The magnified SEM images of nanorod and nanoneedle obtained from scanning electron microscope is shown in inset.
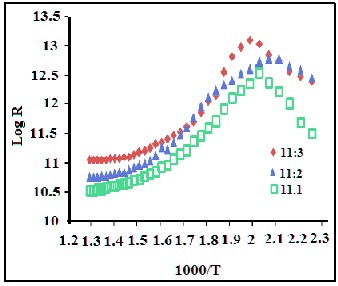
Figure 4(a-c) shows the DC conductivity of barium hexaferrites (Fe/Ba ratio is 11:1, 11:2 and 11:3) sintered at 7500C.
Figure 4 (a-c) shows variation of electrical resistance of barium hexaferrite (Fe/Ba ratio is 11:1, 11:2 and 11:3) pellets of diame- ter 1 cm and thickness 0.5cm with temperature. All barium hexaferrite show almost similar electrical resistance and higher curie temperature. The activation energy of these hexaferrites was calculated by well known Nernst Einstein relation [22]. The activation energy these hexaferrites lies in
7.13-12.1 ×10-5 eV.
IJSER © 2012
International Journal of Scientific & Engineering Research Volume 3, Issue 3, March-2012 4
ISSN 2229-5518
The barium hexaferrite of all Fe/Ba ratios shows high resis- tance. Unlike bulk barium hexaferrite, the difference in curie temperatures was not significant for all. The Curie tempera- ture and activation energy of barium hexaferrite is tabulated in table 1.
Samples | Curie Temp (0K) | Ea x 10-5 eV |
11:1 BaFe12O19 bulk | 280.00 | 7.17 |
11:2 BaFe12O19 bulk | 250.00 | 6.46 |
11:3 BaFe12O19 bulk | 210.00 | 5.43 |
Barium hexaferrite were synthesized for three different Fe/Ba molar ratios 11:1, 11:2 and 11:3. From XRD spectra, is found that for all Fe/Ba ratios, the increase in Ba+ ions concentration in ferrites increases intensity of peaks due to increase in crys- tallinity of the material as expected. The barium hexaferrite calculated by Debye Scherrer’s formula is found to be 26.23 nm, 33.24 nm and 37.86 nm respectively. The FTIR spectra show three important signature absorption peaks of hexafer- rite at ~ 436 cm-1, 546cm-1 and 584cm-1. These peaks confirm the formation barium hexaferrites. The SEM image of barium hexaferrite (Fe/Ba ratio 11:1 & 11:2) at 7500C showed large number of vertically grown barium hexaferrite nanorods de- veloped at some places over the rectangular platelets. The rods having length ~1000nm and diameter ~80nm were developed on the surface of dense rectangular grains. The surface mor- phology of 11:3 barium hexaferrite pellet revealed formation of dense uniform tubular grains as depicted and compara- tively more number of vertically grown long barium hexafer- rite nano-needles having length ~120 nm and base to tip aver- age diameter ~50 nm developed on porous surface of plate like grains of size ~100nm. DC conductivity of barium hexaferrite of all compositions shows almost similar electrical resistance and higher Curie temperature. The activation energy of these hexaferrites lies in 7.13-12.1 ×10-5 eV.
[1] M. Abdullah Dar, Khalid Mujasam Batoo, Vivek Verma, W.A.
Siddiqui, R.K. , Journal of Alloys and Compounds, vol. 493, pp.
553, 2010.
[2] K.H.Maria, S.Choudhary,M.A.Hakim, Journal of Bangladesh
Academy of Sciences,. Vol.34, pp. 1, 2010.
[3] M.H.Khedr, Journal of physicochemical problems of mineral processing, vol.l, no.38, pp. 311, 2004.
[4] D. R. Mane, U. N. Devatwal and K. M. Jadhav, Mat. Lett. Vol.44, pp. 91, 2000.
[5] C. Nlebedim, N. Ranvah, P. I. Williams, Y. Melikhov, F. Anayi, J. E. Synder, A. J. Moses and D. C. Jiles, J. Magn. Magn. Mat. Vol. 321, pp. 2528, 2009.
[6] S. Mahalakshmi, K. S. Manja, J. All. Comp. vol. 457, pp. 522,
2008.
[7] S. A. Mazen, S. F. Mansour, E. Dhahri, H. M. Zaki, A. Elmo- salami, J. All. Comp. vol.470, pp. 294, 2009.
[8] M. A. Elkestawy, J. All. Comp. vol. 492, pp. 616, 2010.
[9] M. El-Shabasy, J. Magn. Magn. Mater. Vol. 172, pp. 188, 2007. [10] R. S. Devan, Y. D. Kolekar, B. K. Chougule, J. Phys. Condens.
Matter. Vol. 18, pp. 9809, 2006.
[11] C. Liu, A. J. Rondinone and Z. J. Zhang, Pure App. Chem, vol.72 , pp. 37, 2000.
[12] L. Liangchao, H. Liua, W. Yuping, J. Jing, X Feng, J. Coll. Int.
Sci., vol. 321, pp. 265, 2008.
[13] M. A. Gabal, Y. M. Al Angari, Mater. Chem. Phys. Vol. 118, pp.
153, 2009.
[14] S. A. Mastia, A. K. Sharmab, P. N. Vasambekarc, A. S. Vain- gankarc, J. Magn. Magn. Mater., vol. 305, pp. 436, 2006.
[15] H. L. Seung, J. Y. Sang, J. L. Gi, S. K. Hong, H. Y. Chul, A.
Kyungsoo, H. L. Dong, H. K. Keu, Mater. Chem. Phy., vol.61, pp. 147, 1999.
[16] Ch. Venkateshwarlu, D. Ravinder, J. All. Comp. vol. 426, pp. 4,
2006.
[17] D. Ravinder, A. C. S. Reddy, J. All. Comp., vol. 353, pp. 86,
2003.
[18] M. M. Masada, M. A. Ahmed, M. A. El-Haiti, S. M. Atria, J.
Magn. Magn. Mater. Vol. 150, pp. 51, 1995.
[19] O. Yamashita, Ener. Conv. Manag. Vol. 50, pp. 1968, 2009.
[20] H.F. Yu, H.Y. Lin, J. Magn. Magn. Mater. Vol. 283, pp. 190, 2004. [21] W. Zhao, W. Ping Wei, H. Cheng, X. Tang, Q. Zhang, J. Am. Ce-
ram. Soc., vol. 90 pp. 2095, 2007.
[22] R.N. Jadhav, D.C. Kulkarni, V. Puri, J. Mater. Sci. Mater. Elec- tron. Vol. 21, pp. 503, 2009.
IJSER © 2012