International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1081
ISSN 2229-5518
Safety of dental units: Efficacy of Chlorine
dioxide in reducing bacterial loads in water and bio films of dental waterlines
Meera S, Sadhana S.
ABSTRACT
OBJECTIVE: To determine efficacy of chlorine dioxide disinfectant in reducing bacterial contamination of dental unit waterlines using bore well / distilled water.
METHODS: Water and biofilm samples were collected from 27 dental unit water lines using bore well water and 20 units using distilled water before the use of, 1 day, 15 days and 30 days after the use of chlorine dioxide (Insta-diox –Narsipur chemicals Pune, Maharashtra). Samples were cultured using nutrient agar and the number of colony forming units was counted using manual colony counting device. Gram’s stain was used to study the type of organisms and M-endo agar for the presence of Escherichia coli.
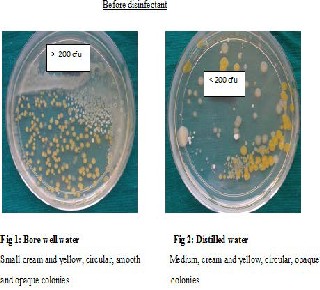
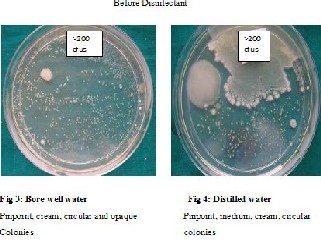
RESULTS: Before disinfection with chlorine dioxide, water and biofilms were highly contaminated. Gram negative bacilli were the most commonly isolated organisms. The number of colony forming units and also the proportion of chairs showing presence of E. coli reduced after disinfection especially 1 day and 15 days after disinfection. All dental unit waterlines met the American Dental Association standards of less than 200/ml colony forming unit until 15 days after disinfection. Bore well water was more contaminated.
INTERPRETATION AND CONCLUSION: Chlorine dioxide is effective in reducing contamination in both water and biofilms.
Key words: dental unit waterlines, colony forming units, chlorine dioxide disinfectant, E.coli, bore well water and distilled water.
—————————— ——————————
Introduction
In any infection control protocol, identifying all possible transmission routes of infectious agents is important1. Dental unit waterlines (DUWLs) delivering water to high speed handpieces, air/water syringes & ultrasonic scalers2, remain a potential weakness in infection control in dental practice, as they become easily contaminated with both patient-derived and input-water impurities3. More than 40 species of microorganisms have been isolated4; many of low pathogenicity, or opportunistic pathogens, causing harmful infections under special conditions/ in immunocompromised persons. Various treatment options investigated to maintain the quality of DUWLs are primarily concerned with bacteria present in the water-borne phase5. Unless procedures specifically designed to prevent/eliminate biofilms are performed, DUWLs would fail to avoid being colonized by bacteria6.
Thus the need for the present study was to assess the bacterial contamination of both output water and the biofilms of DUWLs before the use of, one day after, fifteen days after and one month after the use of chlorine dioxide disinfectant in dental units using bore well water/distilled water.
Materials and methods: 20 ml each of input water –(bore well water and distilled water) was collected before it was run through the DUWLs. Prior to disinfection, output water (after it was run through DUWLs) and biofilm samples were collected from 27 DUWLs using bore well water and 20 DUWLs using distilled water. 20ml of output water samples were collected from three way syringe of dental chair in sterile disposable container. Biofilm samples were collected aseptically from 2 cm long fragment of the tubing from the booster end of dental unit.
The tubings were cut longitudinally and the samples were collected from the internal walls using a sterile cotton swab.
The collected samples of input water, output water and biofilms were each immediately filtered and poured separatelyon the nutrient rich agar and m Endo agar plates and incubated for
48hrs at 37oc. The colony counts were evaluated using manual colony counting device. The type of organisms was studied using Gram’s stain and m Endo agar showed the presence/ absence of E.coli. Following this, 1 liter of prepared chlorine dioxide (Insta-diox) disinfectant solution was filled into the booster bottle at the end of the day, flushed for 2mins and left overnight. Next morning, before the start of any dental procedures the disinfectant from the booster was discarded and refilled with respective water and flushed for 2 minutes. The water and biofilm samples were collected and cultured as above. Bacterial contamination was again evaluated 15 days and 30 days after disinfection in the same manner as mentioned above. A comparison was made between the number of CFUs and type of microorganisms present in water samples and biofilms before the use, one day after, 15 days after and 30 days after the use of chlorine dioxide (Insta-diox) disinfectant between DUWLs using bore well water and those using distilled water. The microbial contamination of the output water was finally compared with the ADA standards
Statistical analysis was done using Chi-Square,Fisher Exact test and t- test
Results: A comparison of Log Colony Forming Units (CFUs) in output water samples of DUWLs using bore well water / distilled water before and after disinfection was done. The log difference of CFUs in water samples before disinfection and 1 day, 15 days and 30 days after disinfection was found to be 2.65,
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1082
ISSN 2229-5518
1.68 and 0.77 respectively in DUWLs using bore well water and
2.63, 1.48 and 0.72 respectively in DUWLs using distilled water (p<0.001 in each of the cases) - Table 1. Similar comparison in biofilms showed that the log difference of CFUs before disinfection and 1 day , 15 days and 30 days after disinfection was 3.24, 1.83 and 0.96 respectively in DUWLs using bore well water and was 3.03, 1.59 and 0.90 respectively in DUWLs using distilled water (p<0.001 in each of the cases) –Table 2.
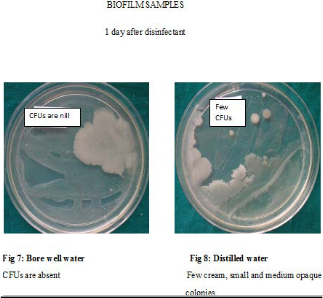
Contamination of output water and biofilms was found to be least on day 1 after disinfection as shown in Table 3
& 4 respectively. The log difference of CFUs in water samples 1
day and 15 days and 30 days after disinfection was -1.11 and -
1.81 respectively for DUWLs using bore well water (p<0.001 in each case) and -0.90 (p<0.039) and -1.72 (p<0.003) respectively for those using distilled water – Table 3. In the biofilms, the log difference of CFUs 1 day and 15 days and 30 days after disinfection was -1.39 and -2.24 respectively for DUWLs using borewell water and -1.41 and -2.12 (p<0.0001 in each case) respectively for those using distilled water – Table 4.
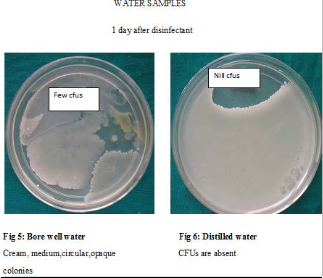
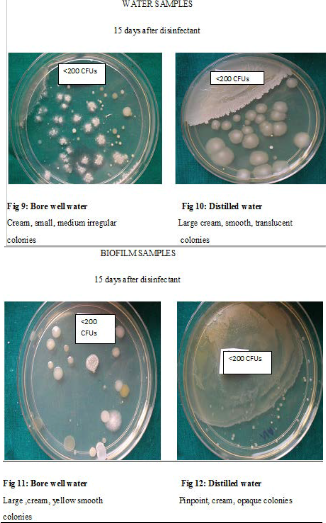
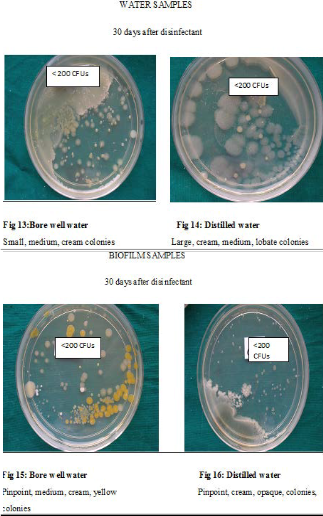
Though the bacterial contamination was reduced after disinfection with chlorine dioxide, it could not be eliminated. Hence the number of DUWLs that maintained the ADA standard of < 200CFUs/ml for dental unit output water, was studied Table 9. Before disinfection, 10/27 DUWLs using bore well water and 5/20 using distilled water failed to maintain the ADA standard. After disinfection with chlorine dioxide, all the DUWLs maintained the standards 1 day after and 15 days after disinfection. After 30 days, only one chair supplied by borewell water showed contamination of more than 200 CFUs/ml.
In the input water,bore well water showed contamination (but maintained potable water standards of <100 CFUs/ml) and distilled water was sterile. However, in the output water, distilled water was also contaminated(Table 1). The mean log CFUs was significantly more in DUWLs (output water) using bore well water than those using distilled water before disinfection (p=0.036). A trend was seen in the same direction 1 day after (p= 0.073+) and 15 days (p= 0.081+) after disinfection following which significantly more number of mean log CFUs was seen in bore well water than in distilled water DUWLs 30 days after disinfection (p= 0.024*). When the biofilms of DUWLs using the bore well water were compared with those using distilled water (Table 2), the mean log CFU was again significantly more in bore well water than in distilled water DUWLs before disinfection (p= 0.047*). There was no significant difference seen 1 day and 15 days after disinfection and only a trend was seen 30 days after disinfection (p= 0.070+) in the same direction as before disinfection.Gram negative bacilli were the most common type of organisms seen in water and biofilm samples Table 5 & 6.
Study of E. coli showed the following results - Before disinfection, a higher proportion of water samples (11/27) and biofilm samples (10/27) of DUWLs using borewell water were contaminated with E coli than those using distilled water (5/20 water samples & 4/20 biofilm samples). However, it was not statistically significant. The same kind of difference in contamination between bore well water and distilled water was not observed after disinfection (1 day/15 days/30 days after). Use of the disinfectant brought about a reduction in the proportion of chairs contaminated with E coli to almost zero level. While
0/20 of the water samples taken from DUWLs using distilled
water were contaminated with E coli 1 day after and 15 day after disinfection (statistical analysis was not possible – Table 7), 3/20
DUWLs were contaminated 30 days after disinfection (p=1.000).
In the DUWLs using borewell water, only 1/27 water samples
was found to be contaminated with E. coli 1 day and 15 days after disinfection (p=0.005) and 3/27 DUWLs were contaminated
30 days after disinfection (p=0.039) (Table 7).
Similar findings were seen even in biofilms (Table 8).
1/20, 0/20 and 2/20 biofilms from DUWLs using distilled water were contaminated with E coli 1 day after , 15 days after and 30 days after disinfection. Because of the small sample size, it is possible that a statistical significance was not obtained on day 1 or 30 days after disinfection. 15 days after disinfection, because of values of zero, statistical analysis was not possible. 0/27, 0/27 and 1/27 biofilms from DUWLs using bore well water were contaminated with E coli 1 day after disinfection, 15 days after disinfection and 30 days (p=0.012) after disinfection.
Discussion: DUWLs that have been in use for several months receiving no decontaminating treatment have been found to be highly contaminated7. In our study, we found significant bacterial contamination in output water samples of DUWLs before disinfection compared to after disinfection (1 day/ 15 days /30 days after disinfection) - Table 1.
Similar findings were obtained on bacterial evaluation
of the biofilms of the DUWLs before and after disinfection - Table 2. This indicates that chlorine dioxide disinfectant used in our study reduced bacterial contamination in both water as well as in the biofilms. In water and in the biofilms, it was found to be most effective on day 1 after disinfection - Table 3 & 4. The results are in agreement with other reports demonstrating the efficacy of a broad range of commercially available treatment products for DUWLs that efficiently remove biofilms and reduce bacterial density in DUWLs8,9,10,11,12,13,14,15,16,5. However, many of these studies have been conducted in vitro and relatively few have actually investigated the efficacy of DUWL treatment products to achieve these desired effects in dental chair units9,13,14,15,16.Our findings also indicate thatthere is recontamination occurring in the DUWLs if not disinfected regularly. Other studies too have shownbiofilm re-growth occurring in DUWLs with subsequent contamination of water in DUWLs usually shortly following disinfection and so DUWLs need to be treated regularly9,13,14,15,11. Different studies have given different time periods before the recontamination takes place. Povidine Iodine (10%) with sterile water reservoirs effectively reduced the CFUs and maintained the ADA standard for 3-14 days17. When DUWLs were treated with Alpron (mixture of Sodium hypochlorite, Citric acid and Sodium-p- toluosulphonechloramide<0.2%), CFUs were reduced to less than the ADA suggested value by the end of 2nd week4, 8 weeks18and 13 week19.
No DUWL can however be completely devoid of microorganisms even after disinfection and thus cannot be called microbiologically “clean”17.
There are currently no official standards or legislations that regulate the microbial quality of DUWL output water. But it is reasonable to expect that the quality of DUWL output water should approximate potable water standards20(Department of health as well as Bureau of Indian Standards (BIS), Government of India set a standard for potable water of 100 CFU/ ml)21 or at least maintain the ADA recommended standard for dental unit
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1083
ISSN 2229-5518
output water of a microbial load of less than 200 CFU/ml 9.We, . therefore, studied the number of DUWLs that maintained / did
not maintain the ADA recommended standard of DUWL output  water after disinfection (Table 9). We found that chlorine
water after disinfection (Table 9). We found that chlorine
dioxide helped to maintain the ADA standard in all DUWLs especially until 15 days after disinfection. Even 30 days after disinfection, only 1 chair failed to maintain the standard. This is in contrast to a study where DUWLs regained the bacterial count (>200 CFUs) after 3 days when disinfected with Chlorine dioxide6.
The quality of DUWL output water is said to be directly influenced by the quality of the supply water18. The bore well input water was contaminated but met the potable water standards. However, the output water was heavily contaminated. Similar observations were made with distilled water. Input distilled water was sterile but output water was heavily contaminated. (Table 1). Irrespective of the kind of water used, the number of CFU/ml coming out of a DUWL is certainly far in excess of the number going in. The inherent multiplication factor between what goes into a DUWL and what comes out in terms of microbial numbers indicates that the bacteria are proliferating in the DUWLs19resulting in build-up of biofilms and subsequent shedding of these biofilms into the water phase22. After assessing the number of CFUs in water samples and biofilms of DUWLs, we also looked at the type of organisms present in the same. In both the water samples (Table 5) and biofilms (Table 6), we found gram positive cocci, gram positive bacillus and gram negative bacillus. Except for day 1 after disinfection when gram positive cocci were commonly isolated, at all other times, it was gram negative bacillus that was most commonly isolated. In literature too, gram negative organisms have been commonly isolated from DUWLs23.
The coliform groups of bacteria have been described as the principal indicators of bacteriological quality of water supplies24. They are also the commonest organisms responsible for water borne diseases in India. Hence,in our study,we looked for the presence of E coli in particular, in both water samples
and biofilms. Before disinfection, a higher proportion of water
samples and biofilm samples of DUWLs using borewell water were contaminated with E coli than those using distilled water though not statistically significant. Use of the disinfectant brought about a reduction in the proportion of chairs contaminated with E coli to almost zero level especially 1 day and 15 days after use of chlorine dioxide (Table 7).
The likelihood of untreated systems providing acceptable water is remote. Contamination in DUWLs is dependent on a number of factors like the quality of input water, handling of reservoir bottles, effectiveness of anti-retraction devices, sterilization of hand pieces, build-up of biofilms which in turn is dependent on number of factors including the number of patients treated, oral status of these patients and the life of the tubings. Hence a single solution for decontaminating dental unit waterlines cannot be recommended.
Conclusion:Chlorine dioxide disinfectant is effective in removal of biofilms from dental unit waterlines.Use of the disinfectant at least once in 15 days is necessary to prevent recontamination. Regular disinfection is a necessary protocol that should be followed in every clinical setting so as to provide a safe working environment for both patients and the dental staff
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1084
ISSN 2229-5518
Acknowledgment: “F. A. Author thanks Dr.Narayan and
Dr.Sadhana Shenoy”
This work was supported in part by the Department of Concervative and periodontics in The Oxford Dental College, Bomannahalli, Bangalore-560068, Karnataka, India.
References:
1. Rodrigues S, Shenoy V and Joseph M. Changing face of infection control: Dental unit water lines. The Journal of Indian Prosthodontic Society. 2005; 5(4): 171.
2. Szymanska J. Control methods of the microbial water quality
in dental unit waterlines. Ann Agric Environ Med. 2003;10(1):1-
4.
3.Franco F, Spratt, Leao J and Porter S. Biofilm formation and control in dental unit waterlines. Biofilms.2005; 2: 9–17.
4. Ritter AV, Ghaname E and Leonard RH. The influence of
dental unit waterline cleaners on composite-to-dentin bonds strengths. J Am Dent Assoc.2007;138(7):985-991.
5. Walker JT, Marsh PD. Microbial biofilm formation in DUWS
and their control using disinfectants. J Dent. 2007;35(9):721-730.
6. Mills SE. The dental unit waterlines controversy: Defusing the myths, defining the solutions. J Am Dent Assoc.
2000;131(10):1427-1441.
7. Nikaeen M, Hatamzadeha M, Sabzevaria Z, Zareha O. Microbial quality of water in dental unit waterlines. JRMS. 2009;
14(5): 297-300.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 2, February-2015 1085
ISSN 2229-5518
8. Montebugnoli LL, Dolci GG. A new chemical formulation for control of dental unit water line contamination: An 'in vitro' and clinical 'study'. BMC Oral Health.2002;2:1-4.
9. Tuttlebee C, Donnell M, Keaney C, Russellx R, Sullivan D,
Falkinerz F and Coleman C. Effective control of dental chair unit waterline biofilm and marked reduction of bacterial contamination of output water using two peroxide-based disinfectants. Journal of Hospital Infection.2002; 52:192-205.
10. Montebugnoli LL, Dolci GG. A new chemical formulation for
control of dental unit water line contamination: An 'in vitro' and clinical 'study'. BMC Oral Health.2002;2:1-4.
11. Smith AJ, Bagg J, Hood J. Use of chlorine dioxide to disinfect
dental unit waterlines. Journal of Hospital Infection.
2001;49:285±288.
12. Martin MV, Gallagher MA. An investigation of the efficacy of super–oxidised (Optident/Sterilox) water for the disinfection of dental unit water lines.British dental journal. 2005
March;198(6):353-354.
13. Walker J, Bradshaw D, FulfordM and Marsh P. Microbiological Evaluation of a Range of Disinfectant Products To Control Mixed-Species Biofilm Contamination in a LaboratoryModel of a Dental Unit Water System. Applied and Environmental Microbiology. 2003 June; 69(6):3327–3332.
14. .Donnell MJ, Shore AC, Coleman DC. A novel automated waterline cleaning system that facilitates effective and consistent control of microbial biofilm contamination of dental chair unit waterlines: A one-year study. Journal of Dentistry. 2006;34:648–
661.
15. Donnell MJ, Shore AC, Russell RJ, Coleman DC. Optimisation of the long-term efficacy of dental chair waterline disinfection by the identification and rectification of factors associated with waterline disinfection failure. Journal of Dentistry. 2007;35:438–451.
16. Szymanska J. Bacterial Decontamination Of Dental unit
waterline biofilm Using Oxygenal 6. Ann Agric Environ Med.
2006;13:163-167.
17. Petti S, TarsitaninG. Detection and Quantification of Dental Unit Water Line Contamination by Oral Streptococci.Infection control and hospital epidemiology.2006; 27: 5-8.
18. Coleman DC, Donnell MJ, Shore AC, Russell RJ. Biofilm
problems in dental unit water systems and its practical control.Journal of Applied Microbiology. 2009;106:1424–1437.
19. Walker JT, Marsha PD. A Review of biofilms and their role in microbial contamination of Dental unit water systems (DUWS). International Biodeterioration and Biodegradation. 2004;54:87-
98.
20. Jeena MI, Deepa P, MujeebRahiman KM, Shanthi RT and Hatha AA. Risk assessment of heterotrophic bacteria from bottled drinking water sold in Indian markets. Int J Hyg Environ Health. 2006 Mar;209(2):191-196.
21. Wdowiak L, Stojek N. Microbial quality of water in dental
unit reservoirs. Ann Agric Environ Med. 2004; 11: 355–358.
22. Marthin MV. The significance of the bacterial contamination of dental unit water systems. Br Dent J. 1987;163:152-4.
23. Lalumandier J, Ayers L. Fluoride and Bacterial Content of
Bottled Water vs Tap Water. Arch Fam Med. 2000;9:246-250.
24. Szymnaska J. Endotoxin level as a potential marker of
concentration of gram-negative bacteria in water effluent from dental units and in dental aerosols. annagric environ med 2005;
12: 229-232.
IJSER © 2015 http://www.ijser.org





