*Corresponding Author
K. Dayananda Reddy, Department of Chemistry, P.V.K.N.Govt. Degree & P.G. College, Chittoor-
517001, A.P., India E-mail: ramanapvkn@gmail.com
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1795
ISSN 2229-5518
L.Venkatramana1, K.Sreenivasulu2, K.Sivakumar,2 K. Dayananda Reddy*1
1 Department of Chemistry, P.V.K.N.Govt. Degree & P.G. College, Chittoor-517001, A.P., India.
2 Department of Chemistry, S.V.Arts Degree & P.G.College (T.T.D’S), Tirupati-517502, A.P., India.
Densities (ρ) of pure liquids and their mixtures have been measured at 298.15 K to 313.15 K and atmospheric pressure over the entire composition range for the binary mixtures of benzyl alcohol with benzene, toluene, chlorobenzene, bromobenzene and nitrobenzene by using Rudolph Research Analytical Digital densitometer (DDH-2911 model). Further, the ultrasonic sound velocities for the above said mixtures were also measured at 303.15 K and 313.15 K. The measured
density data were used to compute excess molar volumes (VE) and these were compared with
Hwang equations. Isentropic compressibility (ks) and excess isentropic compressibilities (k E ) were evaluated from experimental sound velocity and density data. Moreover, the experimental sound velocities were analyzed in terms of theoretical models namely collision factor theory (CFT) and free length theory (FLT). The experimental results were discussed in terms of intermolecular interactions between component molecules.
![]()
*Corresponding Author
K. Dayananda Reddy, Department of Chemistry, P.V.K.N.Govt. Degree & P.G. College, Chittoor-
517001, A.P., India E-mail: ramanapvkn@gmail.com
For many years the chemical industry has recognized the importance of thermodynamic and physical properties in design calculations involving chemical separations, fluid flow, and heat transfer. The study of molecular interaction in the liquid mixtures is of considerable in the
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1796
ISSN 2229-5518
elucidation of the structural properties of the molecules. The intermolecular interactions influence the structural arrangement along with the shape of the molecules. The sign and magnitude of these properties guide us to understand possible interactions between the component molecules [1-4]. Fundamental thermodynamic and thermo physical properties are the essential sources of information that necessary for a better understanding of the non-ideal behavior of complex systems because of physical and chemical effects, which are caused by molecular interactions
,intermolecular forces etc., of unlike molecules. The knowledge of the structure and molecular interactions of liquid mixtures are very important from fundamental and engineering point of view. From a practical point of consideration, these properties are necessary for the development of thermodynamic models required in adequate and optimized processes of the chemical, petro chemical, pharmaceutical, and other industries. In addition, extensive information about structural phenomena of mixtures is of essential importance in development of theories of the liquid state and predictive method. The experimental data on macroscopic properties such as excess molar volumes, excess viscosities, surface tension and refractive index are often useful to understand the nature of homo and hetero molecular interactions, between component molecules. A survey of the literature as shown that excess volume and ultrasonic sound velocity data for the binary mixtures of benzylalcohol with benzene, chlorobenzene, nitrobenzene and benzonitrile were reported at 303.15
K [5]. In the present study, densities (ρ) of pure liquids and their mixtures namely benzylalcohol with benzene, toluene, chlorobenzene bromobenzene, and nitrobenzene were measured over the entire composition range at 298.15 K, 303.15 K, 308.15 K and 313.15 K and ultrasonic sound
velocity data at 303.15 K and 313.15 K. From these data excess volumes (VE) and excess
isentropic compressibility
(k E ) were calculated. Further, the experimental the sound velocity data
were compared with various theories namely collision factor theory (CFT) and free length theory
(FLT). The organic liquids that were chosen in the present investigation are having many industrial applications. Benzyl alcohol is a versatile compound used as a solvent for gelatin, cellulose acetate,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1797
ISSN 2229-5518
shellac and for pharmaceutical aid as an antimicrobial agent [6]. Further, benzyl alcohol is also used in perfumery, in microscopy as an embedding material, and in veterinary applications [7]. Commercial use of benzene and toluene includes synthesis of different intermediate compounds during the process of manufacture of plastics, dyestuffs, detergents and insecticides [8, 9]. The major use of chlorobenzene is an intermediate in the production of commodities such as herbicides, dye stuffs and rubber. Bromobenzene is an important compound in the preparation of Grignard reagent and also an ingredient in the manufacture of phenylcyclidine [10, 11].The largest end use for nitrobenzene is in the production of aniline, p-aminophenol, nigrosine dyes, dyestuffs and resins. The present work was under taken to know the influence of various substituents on benzene ring that may influence both the sign magnitude of excess volume and isentropic compressibility
2.1 Materials
All the chemicals used in the present work were of analytical reagent grade procured from
(S.D.Fine chemicals Ltd.,India and Merck and their purities were as follows: benzyl alcohol
99.7%, benzene 99.5%, toluene 99.5%,chlorobenzene 99.8%, bromobenzene 99.5% and nitrobenzene 99.7% . Prior to experimental measurements, all the liquids were purified as described in the literature [12, 13]. The purity samples were attained by fractional distillation and the purity of chemicals were checked by comparing the measured densities and ultrasonic sound velocity, which were in good agreement with literature values [5, 14-17] and these are given in Table 1. The purity of the sample was further confirmed by GLC single sharp peak. Before use, the chemicals were stored over 0.4nm molecular sieves for about 72hrs to remove water and were later degassed.
2.2 Apparatus and procedure
All the binary liquid mixtures are prepared by weighing an appropriate amount of pure liquids an electronic balance (Afoset, ER –120A, India) with a precision of ±0.1 mg by syringing each
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1798
ISSN 2229-5518
component into airtight stopper bottles to minimize evaporation losses. The uncertainty of the mole fraction was ± 1 × 10-4. After mixing the sample, the bubble free homogenous sample was transferred into the U-tube of the densimeter through a syringe. The density measurements were performed with a Rudolph Research Analytical digital densimeter (DDH-2911 Model), equipped with a built-in solid-state thermostat and a resident program with accuracy of temperature of
303.15 K ±0.03 K.The uncertainty density measurement liquid mixtures are ±2×10–5 gm.cm-3.
Proper calibrations at each temperature were achieved with doubly distilled, deionized water and with air as standards. A multi frequency ultrasonic interferometer (M-82 Model, Mittal Enterprise, New Delhi, India) operated at 2 MHz, was used to measure the ultrasonic velocities of the binary liquid mixtures at temperatures, at 303.15K and 313.15 K by using a digital constant temperature water bath. The uncertainty in the measurement of ultrasonic sound velocity is ±0.3%. The temperature stability is maintained within ± 0.01 K by circulating thermostatic water bath around the cell with a circulating pump. In order to minimize the uncertainty of the measurement, several maxima are allowed to pass and their number (50) in the present study is counted. All maxima are recorded with the highest swing of the needle on the micrometer scale. The total distance d (cm) moved by the reflector is given by d= nλ/2, where λ is the wave length. The frequency, ν, of the
crystal being accurately known (2.0 MHz), the speed of sound, u in ms-1 is calculated by using the
relation u= νλ. The working of the interferometer was tested by making measurements for pure samples of benzyl alcohol, benzene, toluene, chlorobenzene, bromobenzene and nitrobenzene and the measured sound velocities of these liquids are in good agreement which was reported in the literature [18].
The measured densities of pure liquids and their mixtures were given in Table 2 for all the binary mixtures of benzylalcohol with benzene, toluene, chlorobenzene, bromobenzene and nitrobenzene
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1799
ISSN 2229-5518
at temperature range from 298.15 to 313.15 K. The excess molar volume (VE) of all the binary mixtures were calculated from the measured densities using the following equation.
VE/cm3.mol-1 = [x1M1 + x 2M2]/ρm – [x 1M1/ρ1+ x 2M2/ρ2] (1)
where, xi is the mole fraction of component i(i=1,2) in the mixture; Mi is the molar mass ρ and ρi are the measured density of the mixture and the pure component i, respectively. The computed VE data was also given in Table 2 along with predicted in terms of Hwang equation [19].The methods and calculation of VE in terms of Hwang equation were described earlier [20-22]. The VE data for all the binary systems of benzylalcohol with benzene, toluene, chlorobenzene, bromobenzene and
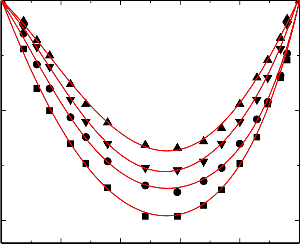
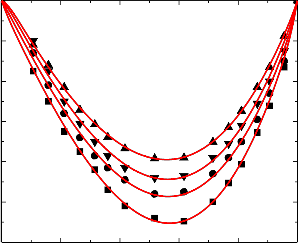
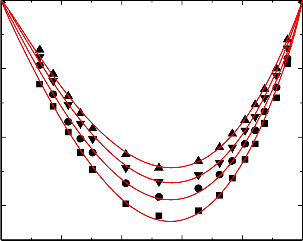
nitrobenzene were graphically represented in Figures 1-5.
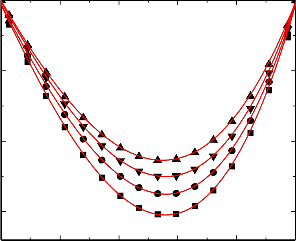
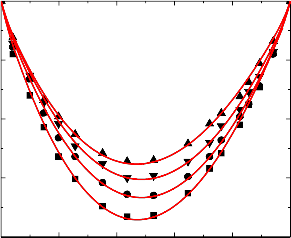
An examination of curves in figures 1-5 shows that, excess volume (VE) data for the binary mixtures benzylalcohol with benzene, toluene, chlorobenzene, bromobenzene and nitrobenzene are negative over the entire composition range at all temperatures. In general, the sign of excess volume (VE) depends on the relative magnitude of contractive and expansive effects that arise on mixing of the component liquids. The factor that causes contraction in volume on mixing are: “Strong specific interactions, generally a kind of chemical interactions, strong physical interactions such as dipole-dipole or dipole-induced dipole interactions and accommodation of molecules of one component in to the interstitial of the structural network of molecules of the other component”. On the other hand, dissociation of one component or both of one components and when the molecular size of the component molecules are very large, which does not favor for fitting of the molecules with each other hand formation of weaker solute-solvent bond than solute-solute and solvent-solvent bonds and these forces are contributing expansion of volume on mixing the
components.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1800
ISSN 2229-5518
An examination of curves in figures from 1-5 indicate that the factors which are responsible for contraction in volume are dominant over the entire composition range for all the binary mixtures. The algebraic excess volume data of all the binary mixtures will fall in the order:
benzene < toluene < chlorobenzene < bromobenzene < nitrobenzene
The above order indicates that extent of negative magnitude of negative excess volume increases due to nature of different substituents that are present on benzene ring.
An examination of VE data in Table 2 for the binary systems benzene and toluene shows that,
more negative VE values is observed in latter case. This is due to introduction of methyl group on benzene ring [23]. So, the electron density on benzene ring increases there by these interactions becomes stronger and this should lead to more negative values of VE benzyl alcohol with toluene. Further, the more negative excess volume data of chlorobenzene when compared to bromobenzene may explained as follows: Chlorobenzene is more reactive than bromobenzene because of the chlorine atom is bonded with sp3 hybridized carbon atom and thereby it can be removed easily. Hence, the rate of reaction of chlorobenzene becomes faster [24-26] when compared to bromobenzene.
The experimental results in the present investigations support this contention. Chlorine atom in chlorobenzene is an electron withdrawing atom, which tries to attract the π-electrons of the benzene ring, there by the electron density of the aromatic ring decreases. As result, the benzene ring in chlorobenzene becomes relatively poor electron donor towards electron seeking proton of any group [27].Hence chlorobenzene interacts strongly with benzylalcohol leading to more vE values.
Further bromobenzene is less reactive when compared to chlorobenzene because of its double bond
character between carbon and bromine atom and also it may be attributed its heavier size. Further,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1801
ISSN 2229-5518
the less negative VE of bromobenzene when compared to chlorobenzene may be postulated in terms of the presence of vacant 3d-orbital in bromine atom of bromobenzene.So that it act as an electron acceptor towards π-electrons of aromatic compounds [28,29]. The more negative VE data for the binary system benzyl alcohol with nitrobenzene when compared to benzene, toluene, chlorobenzene and bromobenzene may be due to its high dipole moment and dielectric constant. Nitrobenzene is supposed to be a relatively complex molecule and its non-ideality arises due to rotation of nitro group freely along the C-N axis where it gives more flexibility to the interaction arising due to the two highly polar N→O bonds [24]. Further, the more negative VE data for the mixture benzylalcohol with nitrobenzene when compared to other mixtures of present investigation may also due to the following reasons: i) Nitro group withdraw the electron cloud from the benzene ring while chloro and bromo groups release the electron cloud to the benzene ring and ii) viscous nature of nitrobenzene[14].
Data for mole fraction (x1) of benzylalcohol density of pure liquids and their liquid mixtures and
experimental sound velocities (u), are included in columns 1-3 of Table 3. Isentropic
compressibilities (κs) and excess isentropic compressibilities (k E )
, were also included in columns
4 and 7 of Table 3.The excess isentropic compressibility data were also represented graphically in
Figures 6-10.The isentropic compresibilities (κS) and excess isentropic compresibilities
(k E )
were
calculated by using the following equations
κS= u-2ρ-1 (2)
The corresponding excess isentropic compressibilities (k E ) were obtained from the relation [30]
κ E κ
κid
(3)
where κ id
is the ideal value of the isentropic compressibility and was calculated from the
following equation [30].
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1802
ISSN 2229-5518
κ id κ
TV α 2 / C
2
x V α
/ x C
(4)
2
s i i1
s.i i i
p.i
T
2
i1
2
i i i i
i1
2
i i1
p.i
Here, Cpi and αi are the molar heat capacity and the thermal expansion coefficient of the ith component respectively. The value of Cpi and αi obtained and evaluated from literature [17,31] and given in Table 4.
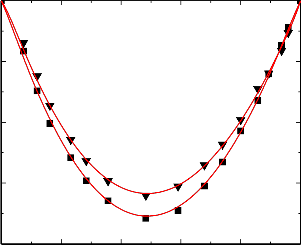
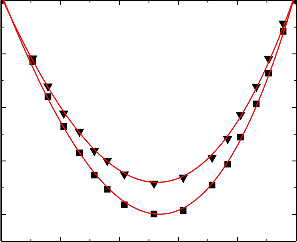
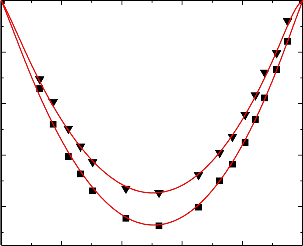
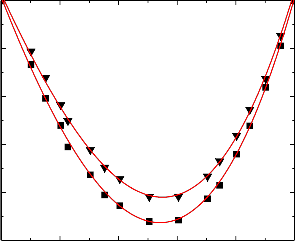
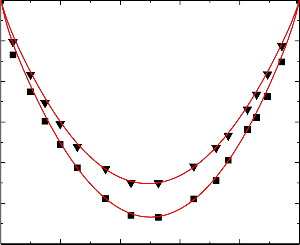
An examination of curves in Figures 6-10 suggest that excess isentropic compressibility data (k E )
for the binary mixtures of benzylalcohol with benzene, toluene, chlorobenzene, bromobenzene and nitrobenzene are negative over the entire composition range at 303.15 K and 313.15 K. This may be interpreted in terms of two opposing effects: i) loss of dipolar association and difference in size and shape of the component molecules; and ii) dipole-dipole, dipole-induced dipole, electron- donor-acceptor interactions and interstitial accommodation of benzylalcohol lattice. The former effect contributes to an increase in free length, described by Jacobson [32]. This leads to negative deviation in sound speed and positive deviation in excess compressibility. The latter effect, on the other hand, leads to positive deviation in sound speed and negative deviation in excess compressibility. The sign and magnitude of the actual deviation depends on the relative strengths of the two opposing effects. As already reported by Benson and Kiyohara [30] the sign and magnitude of the excess isentropic compressibility, that arises through charge transfer, dipole induced dipole and dipole-dipole interactions interstitial accommodation and orientation ordering lead to a more compact structure which contributes to negative excess isentropic compressibility. Our experimental results were also supports this contention.
The algebraic values of κ E values for all the binary systems fall in the order:
Benzene < Toluene < Chlorobenzene < Bromobenzene < Nitrobenzene
The above order indicates that the extent of stronger interactions increases as the free spaces between benzene and substituted benzenes.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1803
ISSN 2229-5518
Experimental ultrasonic sound velocities were analyzed in terms of collision factor theory (CFT) [33], free length theory (FLT) [34,35] and these were also included in Table 3 along with experimental ultrasonic sound velocities. The pure component data namely, the molar volume (Vm), molar volume at absolute zero (V0), molar available volume (Va), free length (Lf), surface area (Y), collision factor(S), average molecular radius (rm), actual volume of molecules per mole (B) and molecular sound velocity(R) that were used to calculate the above said theories were collected from the literature [36]. The methods and details of calculation of theories were discussed earlier [37, 38].
The details of various theories and relevant equations are given as follows:
A comparison between experimental sound velocities and theoretical values suggest that the model proposed by Schaaff’s CFT gives better estimation of sound velocity data. The methods of calculation of these theories were described in earlier. The merits of these theories were compared in terms of relative root mean deviation by using the following formula [39].
RMSD = 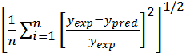 (5) The RMSD for all the binary systems values given in Table 5 shows that Schaaff’s CFT model
(5) The RMSD for all the binary systems values given in Table 5 shows that Schaaff’s CFT model
gives better estimation in sound velocity for the binary mixtures under the investigation.
The experimental VE values and κSE data in have been fitted to Redlich-Kister type polynomial equation[40].
E x x n
a x
x i
y 1 2
i 0 i
1 2
(6)
Where YE= VE or κ E
the subscription ‘i’ in the equation takes value from 0 to 2 ; Ai is the
adjustable parameter of the function and are determined using the least-squares method. The
corresponding standard deviations σ(YE) have been computed using the relation.
σ(YE) =[ Σ(YE
E
cal
)2/(m-n)]1/2 (7)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1804
ISSN 2229-5518
where ‘m’ is the total number of experimental points and ‘n’ is the number of coefficients in
equation 6, and the standard deviations of all the binary mixtures have been presented in Table 6.
In the present work excess volume data of binary mixture of bezylalcohol with benzene, toluene, bromobenzene, chlorobenzene and nitrobenzene were observed the entire composition range from at 298.15 K to 313.15 K and the property is negative in all the binary mixtures.This shows that strong intermolecular interactions are prevailing in liquid mixtures. Since the nitro group of nitrobenzene is more powerful electron withdrawing group when compared to bromobenzene,
chlorobenzene, more negative VE data were observed in the binary system benzylalcohol with
nitrobenzene. Further κ E
data in all the binary mixtures shows that the property is negative at
303.15 K and 313.15 K, which arises due to changes of free volume in the real mixtures and presence of π-electrons in benzylalcohol resulting in the formation of weak intermolecular complexes leading to positive deviation in sound velocity and negative excess isentropic compressibility.
[1] V Syamala, K Sivakumar and P Venkateswarlu, J.Chem.Thermodyn., 38, 1553 (2006) [2]A Ivan, M Ismael, and JA Gonzol, J. Chem. Eng. Data. 55, 5400 (2010)
[3] M Radhamm, P Venkatesh, and MV Prabhakara Rao, J.Chem.,Thermodyn., 40,492 (2008) [4]MV Rathnam, M Sudhir, and MS Kumar, J.Chem.Eng.Data, 55,5946(2010)
[5]A Ali, and M Tariq, J. Mol. Liqs.,128 50 (2006)
[6]W Martindale, The Extra Pharmacopoeia 3rd Edn., Pharmaceutical Press, London (2002). [7]The Merck Index, Merck and Co.Inc, Wiley Interscience, 13th Edn., New York (2001).
[8] D.R.Lide, C.R.C.Hand book of chemistry, 81stEdn, (Boca Raton, New York) (2001).
[9] J.A. Dean, Lange’s Hand book of chemistry, 13th Edn, (McGraw Hill, New York) (1987).
[10] Kirk-Othmer Encyclopedia of Chemical Technology, Wiley Inter science, 5th Edn, 793(2004)
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1805
ISSN 2229-5518
[11] Kirk-Othmer, Encyclopedia of Chemical Technology Wiley Inter science, 5th Edn,224(2004) [12] J A.Riddick, W Bunger and TK Sankano, 4th Edn.,Wiley Interscience, New York(1986). [13]S Timmermans, J.Physico-chemical constants of pure organic compounds, Elsevier,
Amsterdam,(1950)
[14]CL Prabhavathi, K Sivakumar, P Venkateswarulu, and GK Raman, Phys.Chem.Liq.,38,
705(2000)
[15]A Ali, A KNain, D Chand and R Ahmad, J. Mol.Liq, 128, 32 (2006)
[16]J Nayak, I Aralaguppi and TM Aminabhavi, J.Chem. Eng. Data, 48, 628(2003) [17]KS Reddy, R Venkateswralu and GK Raman, Indian J.Chem.Technol., 27, 221(1995) [18]Jaganath and A.P Dixit J. chem. Eng. Data, 29, 313(1984)
[19]CA Hwang, JC Holstc, KR Hall and GA Mansoori, Flu. phase Equ., 62, 173(1991) [20]WE AcreeWilliams, AI Zvaizene and PR Naidu, Phys.Chem.Liq, 27, 69(1994) [21]K Sivakumar and PR Naidu, J. Chem. Eng. Data, 39,2(1994)
[22]V Syamala, P Venkateswarlu, G Prabhakar, and K Sivakumar, J. Phys. Chem. Liq.,44,127 (2006)
[23] S Maken, Ankur Gaur and N Varma, J. Ind. Eng. Chem., 13,1098(2007) [24]S Thirumaran and K Indhu, Rasayan. J. Chemistry, 2, 760(2009)
[25]S Thirumaran and N Karthikeyan, Int. J. Chem.Research, 3,83(2011)
[26]S Thirumaran and E Jayakumar, Indian. J.Pure & App. Phys., 47, 265(2009)
[27]R Acharya, Paikra, and GC Mohanty, Indian J. pure & Appl.Phys.,41, 855(2003). [28]R Tanaka and GC Benson, J.Chem.Eng.Data, 21, 320(1976)
[29]R Tanaka and GC Benson, J.Chem.Eng.Data, 23, 75(1978)
[30]GC Benson, and O Kiyohara, J. Chem. Thermodyn.,11,1061(1979
[31]J Jovanovic, A Knezevic-Stevanovic, D Grozdanic, J. Serb. Chem. Society. 76 (3),417(2011) [32]B Jacobson, Acta. Chem. Scand., 8, 1485 (1952).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1806
ISSN 2229-5518
[33]W Schaffs, Zeitschrift Fur Medizinische Physik .115, 69 (1940) [34]B Jacobson, J. Chem.Phys. 20, 927(1952)
[35]B Jacobson, Acta. Chem. Scand. 8, 1485(1952)
[36]V Syamala, D Rajasekhar, K Sivakumar and P Venkateswarlu, Chin.J.Chem., 25,1(2007) [37]V Syamala,P Venkateswralu and K Sivakumar, J.Chem.Eng.Data, 51, 928(2006)
[38]H Iloukhani, and Z Rostami, J.Sol. Chem ,32 451(2003)
[39]S Mohammad, HAE AlTuwaim, K Alkhaldi, S Adel, and A Abubaker, J.Chem.Thermodyn., 48, 39(2012)
[40]O Redlich, AT Kister,Indian J.Chem.Research. 40, 345(1948)
Figure.1 Variation of excess volume (VE) of the binary liquid mixture of benzylalcohol(1)
with benzene (2) at 298.15 K (▲), 303.15 K (▼), 308.15 K (●) and 313.15 K (■). Figure.2 Variation of excess volume (VE) of the binary liquid mixture of benzylalcohol (1) with toluene (2) at 298.15 K (▲), 303.15 K (▼), 308.15 K (●) and 313.15 K (■).
Figure.3 Variation of excess volume (VE) of the binary liquid mixture of benzylalcohol (1)
with bromobenzene (2) at 298.15 K (▲), 303.15 K (▼), 308.15(●) and 313.15 K (■).
Figure.4 Variation of excess volume (VE) of the binary liquid mixture of benzylalcohol (1)
with chlorobenzene (2) at 298.15 K (▲), 303.15 K (▼), 308.15(●) and 313.15 K (■).
Figure.5 Variation of excess volume (VE) of the binary liquid mixture of benzylalcohol (1)
with nitrobenzene (2) at 298.15 K (▲), 303.15 K (▼), 308.15(●) and 313.15 K (■).
Figure.6 Variation of excess isentropic compressibility (k E ) of the binary liquid mixture of
benzylalcohol (1) with benzene (2) at 303.15 K (▼) and 313.15 K (■).
Figure.7 Variation of excess isentropic compressibility (k E ) of the binary liquid mixture of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1807
ISSN 2229-5518
benzylalcohol (1) with toluene (2) at 303.15 K (▼) and 313.15 K (■).
Figure.8 Variation of excess isentropic compressibility (k E ) of the binary liquid mixture of benzylalcohol (1) with bromobenzene (2) at 303.15 K (▼) and 313.15 K (■).
Figure.9 Variation of excess isentropic compressibility (k E ) of the binary liquid mixture of
benzylalcohol (1) with chlorobenzene (2) at 303.15 K (▼) and 313.15 K (■).
Figure.10 Variation of excess isentropic compressibility (k E ) of the binary liquid mixture of
benzylalcohol (1) with nitrobenzene (2) at 303.15 K (▼) and 313.15 K (■).
Densities ( ρ) and sound velocity (u) of pure components at 303.15 K![]()
Component ρ (g cm-3) u (ms-1)![]()
Present work Literature Present work Literature![]()
Benzyl alcohol 1.03760 1.03700[5] 1514 1511[5] Benzene 0.86855 0.86850[14] 1274 1276[14] Toluene 0.85264 0.85260[15] 1277 1278[15] Bromobenzene 1.48156 1.48150[14] 1140 1138[14]
Chlorobenzene 1.09553 1.09550[16] 1250 1252[14]
Nitrobenzene 1.19345 1.19341[17] 1446 1444[17]![]()
References:Ref:[5], Ref:[14], Ref:[15], Ref:[16], Ref:[17]
Mole fraction of benzylalcohol (x1), densities (ρ), excess volumes (VE) and predicted excess molar volumes (Hwang) at T= 298.15 K to 313.15 K for the binary mixtures of benzylalcohol with
benzene and substituted benzenes
x1 ρ![]()
(gm.cm-3)![]()
VE(Experimental) VE(Hwang)![]()
cm3 mol-1
Benzyl alcohol (1)+Benzene (2) T=298.15 K
0.0743 | 0.88816 | -0.003 | -0.006 |
0.1198 | 0.89688 | -0.016 | -0.013 |
0.1624 | 0.90490 | -0.025 | -0.021 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1808
ISSN 2229-5518
0.2323 | 0.91781 | -0.038 | -0.035 |
0.2837 | 0.92712 | -0.047 | -0.046 |
0.3566 | 0.94005 | -0.057 | -0.059 |
0.4828 | 0.96171 | -0.068 | -0.073 |
0.5904 | 0.97946 | -0.069 | -0.074 |
0.6784 | 0.99351 | -0.064 | -0.066 |
0.7390 | 1.00295 | -0.058 | -0.057 |
0.7997 | 1.01218 | -0.047 | -0.046 |
0.8574 | 1.02080 | -0.035 | -0.033 |
0.8936 | 1.02613 | -0.027 | -0.024 |
0.9362 | 1.03232 | -0.017 | -0.015 |
0.9587 | 1.03551 | -0.006 | -0.009 |
T=303.15 K | |||
0.0743 | 0.88290 | -0.006 | -0.009 |
0.1198 | 0.89170 | -0.021 | -0.018 |
0.1624 | 0.89977 | -0.030 | -0.026 |
0.2323 | 0.91280 | -0.045 | -0.041 |
0.2837 | 0.92220 | -0.055 | -0.052 |
0.3566 | 0.93524 | -0.065 | -0.065 |
0.4828 | 0.95710 | -0.076 | -0.079 |
0.5904 | 0.97502 | -0.077 | -0.082 |
0.6784 | 0.98922 | -0.073 | -0.075 |
0.7390 | 0.99873 | -0.065 | -0.067 |
0.7997 | 1.00808 | -0.055 | -0.055 |
0.8574 | 1.01682 | -0.045 | -0.042 |
0.8936 | 1.02219 | -0.035 | -0.032 |
0.9362 | 1.02842 | -0.022 | -0.020 |
0.9587 | 1.03165 | -0.011 | -0.013 |
T=308.15 K | |||
0.0743 | 0.87768 | -0.015 | -0.018 |
0.1198 | 0.88652 | -0.029 | -0.028 |
0.1624 | 0.89468 | -0.040 | -0.037 |
0.2323 | 0.90779 | -0.053 | -0.049 |
0.2837 | 0.91725 | -0.062 | -0.058 |
0.3566 | 0.93041 | -0.073 | -0.068 |
0.4828 | 0.95246 | -0.084 | -0.080 |
0.5904 | 0.97057 | -0.087 | -0.085 |
0.6784 | 0.98491 | -0.082 | -0.085 |
0.7390 | 0.99454 | -0.076 | -0.080 |
0.7997 | 1.00399 | -0.065 | -0.072 |
0.8574 | 1.01281 | -0.054 | -0.059 |
0.8936 | 1.01826 | -0.046 | -0.049 |
0.9362 | 1.02458 | -0.034 | -0.032 |
0.9587 | 1.02785 | -0.024 | -0.022 |
T=313.15 K | |||
0.0743 | 0.87768 | -0.015 | -0.024 |
0.1198 | 0.87242 | -0.022 | -0.025 |
0.1624 | 0.88137 | -0.040 | -0.035 |
0.2323 | 0.88957 | -0.050 | -0.048 |
0.2837 | 0.90280 | -0.065 | -0.063 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1809
ISSN 2229-5518
0.3566 | 0.91234 | -0.074 | -0.071 |
0.4828 | 0.92561 | -0.085 | -0.082 |
0.5904 | 0.94788 | -0.098 | -0.093 |
0.6784 | 0.96614 | -0.098 | -0.097 |
0.7390 | 0.98061 | -0.093 | -0.094 |
0.7997 | 0.99035 | -0.086 | -0.089 |
0.8574 | 0.99988 | -0.074 | -0.079 |
0.8936 | 1.00880 | -0.062 | -0.065 |
0.9362 | 1.01424 | -0.047 | -0.053 |
0.9587 | 1.02064 | -0.035 | -0.035 |
Benzylalcohol (1)+Toluene (2) T=298.15 K | |||
0.1066 | 0.88221 | -0.016 | -0.020 |
0.1585 | 0.89138 | -0.030 | -0.030 |
0.2116 | 0.90077 | -0.043 | -0.041 |
0.2651 | 0.91026 | -0.055 | -0.050 |
0.3151 | 0.91913 | -0.063 | -0.058 |
0.3593 | 0.92698 | -0.069 | -0.064 |
0.4171 | 0.93725 | -0.075 | -0.071 |
0.5177 | 0.95517 | -0.080 | -0.079 |
0.6169 | 0.97288 | -0.078 | -0.079 |
0.7147 | 0.99036 | -0.069 | -0.073 |
0.7675 | 0.99980 | -0.061 | -0.066 |
0.8111 | 1.00762 | -0.054 | -0.059 |
0.8644 | 1.01717 | -0.043 | -0.046 |
0.9062 | 1.02467 | -0.033 | -0.035 |
0.9556 | 1.03354 | -0.021 | -0.018 |
T=303.15 K | |||
0.1066 | 0.87666 | -0.020 | -0.024 |
0.1585 | 0.88591 | -0.035 | -0.036 |
0.2116 | 0.89540 | -0.050 | -0.047 |
0.2651 | 0.90496 | -0.061 | -0.056 |
0.3151 | 0.91392 | -0.070 | -0.065 |
0.3593 | 0.92185 | -0.077 | -0.075 |
0.4171 | 0.93223 | -0.083 | -0.079 |
0.5177 | 0.95034 | -0.088 | -0.087 |
0.6169 | 0.96825 | -0.087 | -0.088 |
0.7147 | 0.98593 | -0.078 | -0.083 |
0.7675 | 0.99550 | -0.071 | -0.076 |
0.8111 | 1.00340 | -0.062 | -0.068 |
0.8644 | 1.01308 | -0.051 | -0.055 |
0.9062 | 1.02066 | -0.040 | -0.042 |
0.9556 | 1.02963 | -0.025 | -0.022 |
T=308.15 K | |||
0.1066 | 0.87294 | -0.026 | -0.030 |
0.1585 | 0.88219 | -0.042 | -0.043 |
0.2116 | 0.89167 | -0.056 | -0.054 |
0.2651 | 0.90123 | -0.068 | -0.064 |
0.3151 | 0.91018 | -0.077 | -0.073 |
0.3593 | 0.91809 | -0.083 | -0.077 |
0.4171 | 0.92847 | -0.089 | -0.084 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1810
ISSN 2229-5518
0.5177 | 0.94658 | -0.096 | -0.092 |
0.6169 | 0.96448 | -0.095 | -0.095 |
0.7147 | 0.98216 | -0.086 | -0.091 |
0.7675 | 0.99172 | -0.078 | -0.085 |
0.8111 | 0.99962 | -0.070 | -0.077 |
0.8644 | 1.00930 | -0.059 | -0.063 |
0.9062 | 1.01687 | -0.046 | -0.048 |
0.9556 | 1.02583 | -0.030 | -0.026 |
T=313.15 K | |||
0.1066 | 0.86833 | -0.035 | -0.034 |
0.1585 | 0.87761 | -0.050 | -0.051 |
0.2116 | 0.88713 | -0.065 | -0.063 |
0.2651 | 0.89671 | -0.075 | -0.073 |
0.3151 | 0.90570 | -0.084 | -0.082 |
0.3593 | 0.91368 | -0.094 | -0.088 |
0.4171 | 0.92412 | -0.102 | -0.095 |
0.5177 | 0.94231 | -0.108 | -0.103 |
0.6169 | 0.96030 | -0.106 | -0.107 |
0.7147 | 0.97809 | -0.098 | -0.102 |
0.7675 | 0.98770 | -0.089 | -0.096 |
0.8111 | 0.99568 | -0.084 | -0.088 |
0.8644 | 1.00539 | -0.069 | -0.073 |
0.9062 | 1.01300 | -0.055 | -0.056 |
0.9556 | 1.02196 | -0.033 | -0.030 |
Benzylalcohol (1)+ Bromobenzene(2) T=298.15 K | |||
0.1277 | 1.43250 | -0.029 | -0.032 |
0.1722 | 1.41302 | -0.043 | -0.043 |
0.2221 | 1.39109 | -0.056 | -0.055 |
0.2627 | 1.37321 | -0.066 | -0.064 |
0.3032 | 1.35534 | -0.075 | -0.072 |
0.4133 | 1.30651 | -0.090 | -0.089 |
0.5233 | 1.25745 | -0.098 | -0.098 |
0.6544 | 1.19859 | -0.094 | -0.095 |
0.7249 | 1.16678 | -0.086 | -0.087 |
0.7669 | 1.14776 | -0.078 | -0.080 |
0.8091 | 1.12862 | -0.070 | -0.071 |
0.8430 | 1.11321 | -0.061 | -0.062 |
0.8743 | 1.09896 | -0.052 | -0.053 |
0.9137 | 1.08099 | -0.040 | -0.038 |
0.9503 | 1.06423 | -0.023 | -0.023 |
T=303.15 K | |||
0.1277 | 1.42605 | -0.033 | -0.036 |
0.1722 | 1.40671 | -0.047 | -0.047 |
0.2221 | 1.38496 | -0.061 | -0.060 |
0.2627 | 1.36723 | -0.072 | -0.069 |
0.3032 | 1.34949 | -0.081 | -0.077 |
0.4133 | 1.30103 | -0.098 | -0.095 |
0.5233 | 1.25232 | -0.106 | -0.105 |
0.6544 | 1.19385 | -0.102 | -0.103 |
0.7249 | 1.16226 | -0.095 | -0.096 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1811
ISSN 2229-5518
0.7669 | 1.14334 | -0.086 | -0.088 |
0.8091 | 1.12431 | -0.076 | -0.078 |
0.8430 | 1.10900 | -0.068 | -0.069 |
0.8743 | 1.09482 | -0.057 | -0.059 |
0.9137 | 1.07695 | -0.044 | -0.044 |
0.9503 | 1.06030 | -0.028 | -0.027 |
T=308.15 K | |||
0.1277 | 1.41974 | -0.038 | -0.043 |
0.1722 | 1.40058 | -0.055 | -0.055 |
0.2221 | 1.37901 | -0.071 | -0.068 |
0.2627 | 1.36139 | -0.081 | -0.078 |
0.3032 | 1.34376 | -0.089 | -0.085 |
0.4133 | 1.29564 | -0.107 | -0.102 |
0.5233 | 1.24725 | -0.115 | -0.112 |
0.6544 | 1.18915 | -0.110 | -0.112 |
0.7249 | 1.15774 | -0.102 | -0.105 |
0.7669 | 1.13895 | -0.094 | -0.098 |
0.8091 | 1.12003 | -0.084 | -0.088 |
0.8430 | 1.10482 | -0.076 | -0.078 |
0.8743 | 1.09071 | -0.065 | -0.067 |
0.9137 | 1.07294 | -0.051 | -0.051 |
0.9503 | 1.05638 | -0.034 | -0.032 |
T=313.15 K | |||
0.1277 | 1.41354 | -0.049 | -0.050 |
0.1722 | 1.39446 | -0.062 | -0.064 |
0.2221 | 1.37303 | -0.077 | -0.078 |
0.2627 | 1.35556 | -0.089 | -0.087 |
0.3032 | 1.33807 | -0.099 | -0.096 |
0.4133 | 1.29031 | -0.119 | -0.113 |
0.5233 | 1.24222 | -0.126 | -0.124 |
0.6544 | 1.18451 | -0.123 | -0.122 |
0.7249 | 1.15328 | -0.114 | -0.115 |
0.7669 | 1.13459 | -0.104 | -0.107 |
0.8091 | 1.11577 | -0.093 | -0.097 |
0.8430 | 1.10064 | -0.084 | -0.087 |
0.8743 | 1.08661 | -0.072 | -0.075 |
0.9137 | 1.06894 | -0.057 | -0.056 |
0.9503 | 1.05244 | -0.037 | -0.035 |
Benzylalcohol (1)+ Chlorobenzene (2)T=298.15 K | |||
0.1016 | 1.09491 | -0.035 | -0.037 |
0.1512 | 1.09212 | -0.052 | -0.052 |
0.2028 | 1.08918 | -0.066 | -0.065 |
0.2274 | 1.08779 | -0.073 | -0.071 |
0.3037 | 1.08340 | -0.089 | -0.086 |
0.3526 | 1.08057 | -0.097 | -0.094 |
0.4042 | 1.07757 | -0.103 | -0.100 |
0.5044 | 1.07171 | -0.112 | -0.108 |
0.6042 | 1.06580 | -0.111 | -0.109 |
0.7037 | 1.05984 | -0.102 | -0.103 |
0.7446 | 1.05734 | -0.093 | -0.097 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1812
ISSN 2229-5518
0.8028 | 1.05382 | -0.083 | -0.085 |
0.8474 | 1.05106 | -0.069 | -0.073 |
0.9016 | 1.04769 | -0.050 | -0.053 |
0.9529 | 1.04450 | -0.031 | -0.028 |
T=303.15 K | |||
0.1016 | 1.08961 | -0.039 | -0.041 |
0.1512 | 1.08691 | -0.057 | -0.057 |
0.2028 | 1.08405 | -0.071 | -0.071 |
0.2274 | 1.08270 | -0.079 | -0.077 |
0.3037 | 1.07844 | -0.095 | -0.093 |
0.3526 | 1.07570 | -0.105 | -0.102 |
0.4042 | 1.07279 | -0.112 | -0.109 |
0.5044 | 1.06710 | -0.122 | -0.118 |
0.6042 | 1.06134 | -0.122 | -0.121 |
0.7037 | 1.05553 | -0.113 | -0.114 |
0.7446 | 1.05311 | -0.106 | -0.109 |
0.8028 | 1.04963 | -0.092 | -0.096 |
0.8474 | 1.04695 | -0.079 | -0.082 |
0.9016 | 1.04364 | -0.058 | -0.060 |
0.9529 | 1.04049 | -0.035 | -0.032 |
T=308.15 K | |||
0.1016 | 1.08442 | -0.049 | -0.048 |
0.1512 | 1.08179 | -0.065 | -0.065 |
0.2028 | 1.07901 | -0.078 | -0.081 |
0.2274 | 1.07770 | -0.085 | -0.087 |
0.3037 | 1.07361 | -0.105 | -0.103 |
0.3526 | 1.07094 | -0.113 | -0.111 |
0.4042 | 1.06815 | -0.123 | -0.118 |
0.5044 | 1.06264 | -0.133 | -0.127 |
0.6042 | 1.05707 | -0.134 | -0.131 |
0.7037 | 1.05143 | -0.125 | -0.125 |
0.7446 | 1.04907 | -0.117 | -0.120 |
0.8028 | 1.04569 | -0.103 | -0.107 |
0.8474 | 1.04307 | -0.088 | -0.092 |
0.9016 | 1.03986 | -0.067 | -0.068 |
0.9529 | 1.03674 | -0.039 | -0.037 |
T=313.15 K | |||
0.1016 | 1.07920 | -0.056 | -0.054 |
0.1512 | 1.07668 | -0.075 | -0.074 |
0.2028 | 1.07398 | -0.087 | -0.091 |
0.2274 | 1.07274 | -0.097 | -0.098 |
0.3037 | 1.06875 | -0.115 | -0.115 |
0.3526 | 1.06620 | -0.126 | -0.123 |
0.4042 | 1.06348 | -0.135 | -0.130 |
0.5044 | 1.05815 | -0.147 | -0.140 |
0.6042 | 1.05274 | -0.148 | -0.143 |
0.7037 | 1.04725 | -0.138 | -0.138 |
0.7446 | 1.04494 | -0.129 | -0.132 |
0.8028 | 1.04165 | -0.114 | -0.118 |
0.8474 | 1.03909 | -0.098 | -0.103 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1813
ISSN 2229-5518
0.9016 | 1.03594 | -0.075 | -0.076 |
0.9529 | 1.03287 | -0.044 | -0.042 |
Benzyl alcohol(1)+ Nitrobenzene(2)T=298.15 K | |||
0.0409 | 1.19191 | -0.031 | -0.030 |
0.0996 | 1.18304 | -0.066 | -0.065 |
0.1481 | 1.17558 | -0.084 | -0.086 |
0.1988 | 1.16774 | -0.098 | -0.103 |
0.2561 | 1.15219 | -0.113 | -0.116 |
0.3509 | 1.13667 | -0.129 | -0.129 |
0.4356 | 1.12110 | -0.136 | -0.132 |
0.5275 | 1.11289 | -0.135 | -0.129 |
0.6459 | 1.10522 | -0.121 | -0.118 |
0.7207 | 1.09737 | -0.104 | -0.105 |
0.7613 | 1.08926 | -0.095 | -0.097 |
0.8257 | 1.08205 | -0.081 | -0.079 |
0.8562 | 1.07335 | -0.069 | -0.069 |
0.8934 | 1.06522 | -0.053 | -0.054 |
0.9409 | 1.05200 | -0.034 | -0.033 |
T=303.15 K | |||
0.0409 | 1.18705 | -0.036 | -0.032 |
0.0996 | 1.17815 | -0.063 | -0.069 |
0.1481 | 1.17081 | -0.086 | -0.091 |
0.1988 | 1.16307 | -0.104 | -0.109 |
0.2561 | 1.14767 | -0.123 | -0.123 |
0.3509 | 1.13226 | -0.138 | -0.136 |
0.4356 | 1.11685 | -0.15 | -0.141 |
0.5275 | 1.10869 | -0.148 | -0.140 |
0.6459 | 1.10110 | -0.136 | -0.131 |
0.7207 | 1.09331 | -0.120 | -0.119 |
0.7613 | 1.08520 | -0.106 | -0.111 |
0.8257 | 1.07804 | -0.092 | -0.092 |
0.8562 | 1.06937 | -0.077 | -0.081 |
0.8934 | 1.06133 | -0.064 | -0.065 |
0.9409 | 1.04818 | -0.043 | -0.040 |
T=308.15 K | |||
0.0409 | 1.18218 | -0.039 | -0.035 |
0.0996 | 1.17335 | -0.066 | -0.074 |
0.1481 | 1.16613 | -0.095 | -0.099 |
0.1988 | 1.15848 | -0.116 | -0.119 |
0.2561 | 1.14316 | -0.132 | -0.135 |
0.3509 | 1.12793 | -0.154 | -0.151 |
0.4356 | 1.11261 | -0.164 | -0.156 |
0.5275 | 1.10454 | -0.165 | -0.155 |
0.6459 | 1.09696 | -0.149 | -0.145 |
0.7207 | 1.08921 | -0.132 | -0.132 |
0.7613 | 1.08116 | -0.118 | -0.122 |
0.8257 | 1.07398 | -0.098 | -0.101 |
0.8562 | 1.06541 | -0.087 | -0.089 |
0.8934 | 1.05740 | -0.072 | -0.071 |
0.9409 | 1.04428 | -0.045 | -0.043 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1814
ISSN 2229-5518
T=313.15 K | |||
0.0409 | 1.17735 | -0.045 | -0.040 |
0.0996 | 1.16867 | -0.080 | -0.086 |
0.1481 | 1.16148 | -0.107 | -0.115 |
0.1988 | 1.15393 | -0.132 | -0.137 |
0.2561 | 1.13876 | -0.151 | -0.155 |
0.3509 | 1.12364 | -0.174 | -0.172 |
0.4356 | 1.10842 | -0.183 | -0.177 |
0.5275 | 1.10038 | -0.182 | -0.173 |
0.6459 | 1.09282 | -0.163 | -0.157 |
0.7207 | 1.08509 | -0.142 | -0.141 |
0.7613 | 1.07710 | -0.129 | -0.129 |
0.8257 | 1.06994 | -0.105 | -0.105 |
0.8562 | 1.06137 | -0.088 | -0.091 |
0.8934 | 1.05341 | -0.073 | -0.072 |
0.9409 | 1.04036 | -0.044 | -0.043 |
![]()
Molefraction(x1) of benzylalcohol, densities (ρ), sound velocities(u), isentropic compressibilities
(κs ), excess isentropic compressibilities
(k E )
and theoretical sound velocity values of
benzylalcohol (1) with benzene and substituted benzenes(2) at 303.15K and 313.15 K.![]()
(Exp)![]()
Benzylalcohol (1)+Benzene (2) T=303.15 K
s
(m.s-1)
0.0743 0.88290 1299 671 1297 1301 -13.8
0.1198 0.89170 1313 651 1305 1311 -24.8
0.1624 0.89977 1327 631 1324 1327 -34.5
0.2323 0.91280 1348 603 1327 1338 -45.7
0.2837 0.92220 1366 581 1338 1351 -54.3
0.3566 0.93524 1388 555 1353 1369 -60.9
0.4828 0.95710 1423 516 1381 1397 -64.1
0.5904 0.97502 1452 486 1406 1422 -61.6
0.6784 0.98922 1471 467 1428 1442 -54.1
0.739 0.99873 1483 455 1443 1456 -47.3
0.7997 1.00808 1494 444 1459 1469 -39.2
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1815
ISSN 2229-5518
0.8574 | 1.01682 | 1501 | 437 | 1474 | 1482 | -29.0 |
0.8936 | 1.02219 | 1508 | 430 | 1484 | 1491 | -23.9 |
0.9362 | 1.02842 | 1514 | 424 | 1496 | 1500 | -16.4 |
0.9587 | 1.03165 | 1518 | 421 | 1502 | 1505 | -12.9 |
T=313.15 K | ||||||
0.0743 | 0.87242 | 1305 | 673 | 1294 | 1316 | -16.6 |
0.1198 | 0.88137 | 1321 | 650 | 1302 | 1326 | -29.7 |
0.1624 | 0.88957 | 1336 | 630 | 1320 | 1342 | -40.4 |
0.2323 | 0.90280 | 1357 | 602 | 1328 | 1355 | -51.7 |
0.2837 | 0.91234 | 1374 | 580 | 1346 | 1372 | -59.3 |
0.3566 | 0.92561 | 1396 | 554 | 1381 | 1401 | -65.9 |
0.4828 | 0.94788 | 1435 | 512 | 1403 | 1426 | -71.6 |
0.5904 | 0.96614 | 1465 | 482 | 1421 | 1446 | -69.1 |
0.6784 | 0.98061 | 1484 | 463 | 1432 | 1460 | -61.0 |
0.739 | 0.99035 | 1495 | 451 | 1447 | 1474 | -53.1 |
0.7997 | 0.99988 | 1503 | 442 | 1461 | 1487 | -42.9 |
0.8574 | 1.00880 | 1511 | 434 | 1468 | 1495 | -32.9 |
0.8936 | 1.01424 | 1512 | 431 | 1480 | 1504 | -24.1 |
0.9362 | 1.02064 | 1515 | 427 | 1484 | 1509 | -14.7 |
0.9587 | 1.02397 | 1515 | 425 | 1472 | 1514 | -8.8 |
Benzylalcohol (1)+Toluene (2) T=303.15 K | ||||||
0.1066 | 0.87666 | 1307 | 668 | 1306 | 1309 | -21.6 |
0.1585 | 0.88591 | 1325 | 643 | 1315 | 1320 | -32.1 |
0.2116 | 0.89540 | 1345 | 617 | 1325 | 1332 | -42.2 |
0.2651 | 0.90496 | 1364 | 594 | 1335 | 1343 | -49.0 |
0.3151 | 0.91392 | 1383 | 572 | 1345 | 1354 | -56.2 |
0.3593 | 0.92185 | 1400 | 553 | 1353 | 1364 | -59.9 |
0.4171 | 0.93223 | 1418 | 553 | 1365 | 1377 | -66.1 |
0.5177 | 0.95034 | 1451 | 500 | 1387 | 1399 | -68.4 |
0.6169 | 0.96825 | 1477 | 473 | 1410 | 1422 | -67.3 |
0.7147 | 0.98593 | 1495 | 454 | 1434 | 1445 | -58.7 |
0.7675 | 0.99550 | 1500 | 446 | 1448 | 1457 | -51.7 |
0.8111 | 1.00340 | 1504 | 441 | 1460 | 1468 | -42.8 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1816
ISSN 2229-5518
0.8644 | 1.01308 | 1508 | 434 | 1474 | 1481 | -32.2 |
0.9062 | 1.02066 | 1506 | 432 | 1486 | 1491 | -21.8 |
0.9556 | 1.02963 | 1505 | 429 | 1500 | 1503 | -8.6 |
T=313.15 K | ||||||
0.1066 | 0.86833 | 1317 | 663 | 1290 | 1316 | -22.9 |
0.1585 | 0.87761 | 1336 | 638 | 1299 | 1327 | -36.0 |
0.2116 | 0.88713 | 1356 | 613 | 1309 | 1339 | -47.1 |
0.2651 | 0.89671 | 1374 | 591 | 1320 | 1351 | -57.0 |
0.3151 | 0.90570 | 1393 | 569 | 1329 | 1362 | -65.3 |
0.3593 | 0.91368 | 1409 | 551 | 1338 | 1371 | -70.6 |
0.4171 | 0.92412 | 1428 | 531 | 1350 | 1384 | -76.4 |
0.5177 | 0.94231 | 1459 | 499 | 1372 | 1407 | -81.0 |
0.6169 | 0.96030 | 1485 | 477 | 1394. | 1429 | -79.7 |
0.7147 | 0.97809 | 1503 | 453 | 1417 | 1452 | -69.0 |
0.7675 | 0.98770 | 1511 | 443 | 1430 | 1464 | -61.2 |
0.8111 | 0.99568 | 1512 | 439 | 1441 | 1474 | -51.1 |
0.8644 | 1.00539 | 1514 | 434 | 1455 | 1486 | -38.7 |
0.9062 | 1.01300 | 1513 | 431 | 1466 | 1496 | -27.2 |
0.9556 | 1.02196 | 1509 | 429 | 1480 | 1508 | -11.6 |
Benzylalcohol (1)+ Bromobenzene(2) T=303.15 K | ||||||
0.1277 | 1.42605 | 1187 | 497 | 1048 | 1187 | -30.5 |
0.1722 | 1.40671 | 1213 | 483 | 1064 | 1204 | -39.3 |
0.2221 | 1.38496 | 1242 | 468 | 1084 | 1223 | -49.7 |
0.2627 | 1.36723 | 1267 | 456 | 1100 | 1238 | -56.6 |
0.3032 | 1.34949 | 1291 | 445 | 1117 | 1253 | -63.6 |
0.4133 | 1.30103 | 1359 | 416 | 1165 | 1294 | -73.9 |
0.5233 | 1.25232 | 1414 | 399 | 1217 | 1335 | -75.8 |
0.6544 | 1.19385 | 1466 | 390 | 1286 | 1384 | -68.9 |
0.7249 | 1.16226 | 1486 | 391 | 1327 | 1411 | -61.2 |
0.7669 | 1.14334 | 1493 | 392 | 1352 | 1426 | -54.1 |
0.8091 | 1.12431 | 1498 | 396 | 1379 | 1442 | -44.3 |
0.8430 | 1.10900 | 1502 | 400 | 1401 | 1455 | -36.7 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1817
ISSN 2229-5518
0.8743 | 1.09482 | 1503 | 404 | 1422 | 1467 | -27.9 |
0.9137 | 1.07695 | 1505 | 410 | 1450 | 1481 | -16.3 |
0.9503 | 1.06030 | 1506 | 416 | 1476 | 1495 | -7.0 |
T=313.15 K | ||||||
0.1277 | 1.41354 | 1193 | 493 | 1039 | 1194 | -34.2 |
0.1722 | 1.39446 | 1217 | 480 | 1055 | 1211 | -48.0 |
0.2221 | 1.37303 | 1246 | 465 | 1075 | 1231 | -60.5 |
0.2627 | 1.35556 | 1271 | 453 | 1091 | 1246 | -67.3 |
0.3032 | 1.33807 | 1296 | 441 | 1107 | 1262 | -73.9 |
0.4133 | 1.29031 | 1364 | 413 | 1155 | 1303 | -84.5 |
0.5233 | 1.24222 | 1421 | 395 | 1206 | 1345 | -87.5 |
0.6544 | 1.18451 | 1476 | 384 | 1274 | 1394 | -80.2 |
0.7249 | 1.15328 | 1498 | 383 | 1313 | 1420 | -70.0 |
0.7669 | 1.13459 | 1504 | 387 | 1338 | 1435 | -63.5 |
0.8091 | 1.11577 | 1509 | 391 | 1364 | 1450 | -55.2 |
0.843 | 1.10064 | 1510 | 395 | 1385 | 1462 | -46.2 |
0.8743 | 1.08661 | 1511 | 400 | 1405 | 1474 | -37.8 |
0.9137 | 1.06894 | 1512 | 406 | 1431 | 1488 | -26.8 |
0.9503 | 1.05244 | 1512 | 413 | 1457 | 1501 | -15.8 |
Benzyl alcohol (1)+ Chlorobenzene(2) T=303.15 K | ||||||
0.1016 | 1.08961 | 1292 | 550 | 1273 | 1275 | -21.1 |
0.1512 | 1.08691 | 1313 | 534 | 1285 | 1287 | -32.1 |
0.2028 | 1.08405 | 1338 | 515 | 1298 | 1301 | -43.5 |
0.2274 | 1.08270 | 1351 | 506 | 1304 | 1307 | -50.1 |
0.3037 | 1.07844 | 1386 | 483 | 1323 | 1326 | -62.2 |
0.3526 | 1.07570 | 1409 | 468 | 1335 | 1339 | -69.6 |
0.4042 | 1.07279 | 1433 | 454 | 1348 | 1352 | -76.9 |
0.5044 | 1.06710 | 1474 | 431 | 1374 | 1379 | -83.9 |
0.6042 | 1.06134 | 1506 | 415 | 1401 | 1405 | -82.4 |
0.7037 | 1.05553 | 1528 | 406 | 1428 | 1432 | -75.1 |
0.7446 | 1.05311 | 1532 | 405 | 1439 | 1443 | -67.0 |
0.8028 | 1.04963 | 1540 | 401 | 1456 | 1459 | -56.3 |
0.8474 | 1.04695 | 1537 | 404 | 1468 | 1471 | -45.4 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1818
ISSN 2229-5518
0.9016 | 1.04364 | 1533 | 407 | 1484 | 1486 | -28.2 |
0.9529 | 1.04049 | 1470 | 445 | 1499 | 1500 | -14.6 |
T=313.15 K | ||||||
0.1016 | 1.07920 | 1306 | 543 | 1224 | 1258 | -26.7 |
0.1512 | 1.07668 | 1328 | 527 | 1220 | 1260 | -40.8 |
0.2028 | 1.07398 | 1355 | 507 | 1216 | 1262 | -52.1 |
0.2274 | 1.07274 | 1366 | 500 | 1214 | 1262 | -61.1 |
0.3037 | 1.06875 | 1402 | 476 | 1207 | 1265 | -72.7 |
0.3526 | 1.06620 | 1424 | 463 | 1204 | 1267 | -81.1 |
0.4042 | 1.06348 | 1448 | 448 | 1199 | 1269 | -85.6 |
0.5044 | 1.05815 | 1490 | 426 | 1192 | 1272 | -92.2 |
0.6042 | 1.05274 | 1523 | 410 | 1184 | 1275 | -91.6 |
0.7037 | 1.04725 | 1549 | 398 | 1176 | 1279 | -82.7 |
0.7446 | 1.04494 | 1555 | 396 | 1173 | 1280 | -77.1 |
0.8028 | 1.04165 | 1559 | 395 | 1168 | 1281 | -64.1 |
0.8474 | 1.03909 | 1557 | 396 | 1165 | 1283 | -52.3 |
0.9016 | 1.03594 | 1550 | 402 | 1161 | 1284 | -36.2 |
0.9529 | 1.03287 | 1541 | 408 | 1157 | 1285 | -18.9 |
Benzyl alcohol (1) + Nitrobenzene(2) T=303.15 K | ||||||
0.0409 | 1.18705 | 1480 | 385 | 1448 | 1448 | -20.6 |
0.0996 | 1.17815 | 1512 | 371 | 1451 | 1452 | -36.6 |
0.1481 | 1.17081 | 1539 | 361 | 1454 | 1455 | -50.5 |
0.1988 | 1.16307 | 1561 | 353 | 1457 | 1459 | -61.0 |
0.2561 | 1.14767 | 1590 | 345 | 1464 | 1463 | -72.2 |
0.3509 | 1.13226 | 1618 | 337 | 1471 | 1469 | -81.8 |
0.4356 | 1.11685 | 1633 | 336 | 1478 | 1475 | -88.2 |
0.5275 | 1.10869 | 1639 | 336 | 1480 | 1481 | -88.8 |
0.6459 | 1.10110 | 1628 | 343 | 1481 | 1489 | -81.8 |
0.7207 | 1.09331 | 1613 | 352 | 1484 | 1494 | -72.7 |
0.7613 | 1.08520 | 1603 | 359 | 1488 | 1497 | -66.6 |
0.8257 | 1.07804 | 1583 | 370 | 1491 | 1501 | -53.7 |
0.8562 | 1.06937 | 1578 | 376 | 1496 | 1504 | -46.4 |
0.8934 | 1.06133 | 1565 | 385 | 1500 | 1506 | -36.4 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1819
ISSN 2229-5518
0.9409 | 1.04818 | 1551 | 396 | 1508 | 1509 | -22.5 |
T=313.15 K | ||||||
0.0409 | 1.17735 | 1490 | 383 | 1410 | 1443 | -26.9 |
0.0996 | 1.16867 | 1535 | 363 | 1391 | 1435 | -45.1 |
0.1481 | 1.16148 | 1561 | 353 | 1376 | 1427 | -59.6 |
0.1988 | 1.15393 | 1585 | 345 | 1360 | 1419 | -71.1 |
0.2561 | 1.13876 | 1615 | 337 | 1346 | 1410 | -82.6 |
0.3509 | 1.12364 | 1644 | 329 | 1319 | 1396 | -97.8 |
0.4356 | 1.10842 | 1663 | 326 | 1297 | 1382 | -103.4 |
0.5275 | 1.10038 | 1664 | 328 | 1268 | 1367 | -104.8 |
0.6459 | 1.09282 | 1643 | 339 | 1232 | 1348 | -97.9 |
0.7207 | 1.08509 | 1630 | 347 | 1211 | 1335 | -88.8 |
0.7613 | 1.07710 | 1624 | 352 | 1202 | 1328 | -78.8 |
0.8257 | 1.06994 | 1601 | 365 | 1186 | 1317 | -63.7 |
0.8562 | 1.06137 | 1593 | 371 | 1181 | 1312 | -57.8 |
0.8934 | 1.05341 | 1581 | 380 | 1173 | 1305 | -47.4 |
0.9409 | 1.04036 | 1562 | 394 | 1166 | 1297 | -30.4 |
![]()
Table 4 Thermal coefficient (α) and heat capacity (cp) for pure component liquids at temperatures
303.15K and 313.15 K.
![]()
Component/Temperature 303.15 313.15![]()
Benzyl alcohol
α (kK-1) 0.7402 0.7456
Cp (J.mol-1.k-1) 224.35[17] 227.62[32] Benzene
α (kK-1) 1.1915 1.2048
Cp (J.mol-1.k-1) 137.4[17] 140.6[32] Toluene
α (kK-1) 1.2666 1.0612
Cp (J.mol-1.k-1) 153.4[17] 160.2[32] Bromobenzene
α (kK-1) 0.9048 0.8785
Cp (J.mol-1.k-1) 156.9[17] 157.3[32] Chlorobenzene
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1820
ISSN 2229-5518
α (kK-1) | 0.9725 | 0.9673 |
Cp (J.mol-1.k-1) | 150.6[17] | 152.2[32] |
α (kK-1) | Nitrobenzene 0.8198 | 0.8217 |
Cp (J.mol-1.k-1) | 177.3[17] | 173.8[32] |
![]()
RMSD of speed of sound (u) of benzylalcohol with benzene and substituted benzene at T=
303.15K and 313.15 K from different relations![]()
RMSD 303.15 K 313.15 K![]()
![]()
![]()
Benzyl alcohol (1) + benzene (2)
CFT | 0.013 | 0.008 |
FLT | 0.020 | 0.023 |
Benzyl alcohol (1) + toluene(2) | ||
CFT | 0.023 | 0.024 |
FLT CFT | 0.029 Benzyl alcohol (1) + bromobenzene(2) 0.035 | 0.045 0.034 |
FLT | 0.107 | 0.119 |
CFT | Benzyl alcohol(1) + chlorobenzene(2) 0.046 | 0.137 |
FLT | 0.048 Benzyl alcohol (1) + nitrobenzene(2) | 0.194 |
CFT | 0.066 | 0.151 |
FLT | 0.068 | 0.209 |
![]()
Coefficients Ai of Redlich-Kister equation 6 and the corresponding standard deviations (σ) of all
the systems
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1821
ISSN 2229-5518
![]()
Temperature Function A0 A1 A2 σ(VE)![]()
Benzyl alcohol (1)+benzene (2)
289.15 K VE -0.295 -0.088 0.178 0.003
303.15 K VE -0.323 -0.111 0.114 0.003
308.15 K VE -0.325 -0.146 -0.102 0.004
313.15 K VE -0.376 -0.118 -0.131 0.003
303.15 K κsE -252.1 -252.1 -1.55 2.9
313.15 K κsE
-294.3 12.6 55.82 1.3
Benzyl alcohol (1)+Toulene(2)
289.15 K VE -0.311 -0.115 0.002 0.004
303.15 K VE -0.342 -0.135 -0.044 0.004
308.15 K VE -0.364 -0.144 -0.124 0.005
313.15 K VE -0.408 -0.153 -0.181 0.004
303.15 K κsE -284.6 -20.77 -20.77 1.9
313.15 K κsE -332.6 -44.61 88.15 1.4
Benzyl alcohol (1)+bromobenzene (2)
289.15 K VE -0.388 -0.123 0.014 0.002
303.15 K VE -0.414 -0.138 -0.025 0.002
308.15 K VE -0.440 -0.147 -0.107 0.004
313.15 K VE -0.484 -0.147 -0.145 0.003
303.15 K κsE -327.6 11.13 147.19 3.2
313.15 K κsE -357.5 -13.60 41.63 1.2
Benzyl alcohol (1)+chlorobenzene (2)
289.15 K VE -0.434 -0.111 -0.105 0.003
303.15 K VE -0.472 -0.138 -0.142 0.003
308.15 K VE -0.510 -0.144 -0.214 0.003
313.15 K VE -0.560 0.152 -0.269 0.003
303.15 K κsE -338.6 -65.97 87.86 1.2
313.15 K κsE -373.47 -67.21 24.1 1.2
Benzyl alcohol (1)+nitrobenzene (2) | |||||
289.15 K | VE | -0.523 | 0.086 | -0.196 | 0.003 |
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1822
ISSN 2229-5518
303.15 K VE
308.15 K VE
313.15 K VE
303.15 K κsE
313.15 K κsE![]()
0.00
-0.05
-0.10
0.0 0.2 0.4 0.6 0.8 1.0
X
1
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1823
ISSN 2229-5518
0.00
-0.02
-0.04
-0.06
-0.08
-0.10
-0.12
0.0 0.2 0.4 0.6 0.8 1.0
X
1
0.00
-0.04
-0.08
-0.12
0.0 0.2 0.4 0.6 0.8 1.0
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1824
ISSN 2229-5518
0.00
-0.05
-0.10
-0.15
0.0 0.2 0.4 0.6 0.8 1.0
X
1
0.00
-0.05
-0.10
-0.15
-0.20
0.0 0.2 0.4 0.6 0.8 1.0
1
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1825
ISSN 2229-5518
0
-20
-40
-60
-80
0.0 0.2 0.4 0.6 0.8 1.0
x1
0
-20
-40
-60
-80
0.0 0.2 0.4 0.6 0.8 1.0
x1
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1826
ISSN 2229-5518
0
-20
-40
-60
-80
0.0 0.2 0.4 0.6 0.8 1.0
x1
0
-20
-40
-60
-80
-100
0.0 0.2 0.4 0.6 0.8 1.0
x1
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1827
ISSN 2229-5518
0
-20
-40
-60
-80
-100
-120
0.0 0.2 0.4 0.6 0.8 1.0
x1
IJSER © 2013 http://www.ijser.org