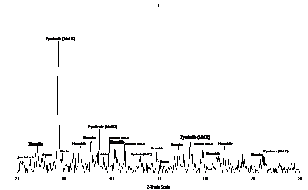International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 339
ISSN 2229-5518
Pelletization and Reduction of Egyptian Low Grade Manganese Ore Pellets via Hydrogen at 750-950ºC
El-Hussiny N.A.(1) , Hala H. Abd El-Gawad(2), Mohamed F.M.(1,3), Shalabi M.E.H.(1*)
1-Centeral Metallurgical Research and Development Institute, (CMRDI), Cairo- Egypt
2-Faculty of Science and arts Mohail Asser king khalid university, Saudi Arabia
3- King Khalid University, Faculty of science and Arts For Girls. Sarat Abida . Saudi Arabia
* Corresponding author: e.mail : shalabimeh@hotmail.com
Abstract: Egyptian low grade manganese ore pellets were reduced by hydrogen in this work in the temperature range
750-950ºC. The results indicated that the reduction rate increased with temperature rise. And it was found that the reaction model is: - ln(1-R) = kt , and the energy of activation is energy 79.78 kJ/mole.
Key Word: Pelletization process, reduction of pellets via hydrogen, reduction kinetic models
Introduction
—————————— ——————————
V. kivinen, et al[7] indicated that huge amounts of low-grade manganese ores are located in many
Manganese is a strategic element and plays an important role in several industrial applications, such as steel production as an alloying element, it improves the strength, toughness, hardenability, workability and abrasion resistance of the ferrous products,, preparation of dietary additives, , carbon– zinc batteries production, fertilizers, cells and fine chemicals, as well as colorants for bricks, dyes and medicines [1-5]
The world annual consumption of manganese is above 1,300,000 annual tons and it is destined to increase. Low grade ores are gaining increasing attention due to developments in exploitation technologies [2]. About 90 – 95 of all the manganese produced in the world is used in iron and steel production in the form of alloys such as ferromanganese and silicomanganese. Manganese has two important properties in steelmaking: its ability to combine with sulphur to form MnS and it’s deoxidation capacity. Today about 30% of the manganese used in steel industry for its properties as a sulphide former and deoxidant. The other 70% of the manganese is used purely as an alloying element [6]
countries around the world. These deposits are only partially used in ferromanganese production. The ores contain large quantities of iron and thus the Mn / Fe ratio is low from 3 - 4. Iron appears partially in ore as individual hematite and goethite grains and the remainder of it is in the manganese grains. The laboratory tests showed that the Mn / Fe ratio increased up to 11 when the pre-reduction of ore was performed in a laboratory rotary kiln at about 800°C. The pre-reduction was performed in a reducing gas atmosphere and with solid carbon. The product from the kiln contained individual magnetite grains, which were separated after grinding by a magnetic separation method. The non-magnetic fraction requires pelletizing and sintering in a separate process step to produce durable and porous pellets, which are excellent raw material for ferromanganese smelting
Yubo Gao [5] illustrated that because of intensive mining of high-grade manganese ores for a long time while leaving behind the low-grade ores, the utilization of the latter has become necessary. There
are several physicochemical differences among the
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 340
ISSN 2229-5518
components in manganese ores, which can be used for the enrichment of manganese. In particular, the abundant low-grade manganese ores, which contain iron oxide, may be upgraded by pre-reduction and magnetic separation. It was pre-reduced, ferruginous low-grade manganese ore by CO, which converted iron oxide to Fe3 O4 , while manganese oxide was reduced to MnO. Then, the iron-rich component was collected by magnetic separation. From the kinetics study, it was found that the reduction rate is increased by an increase in the temperature and in CO content of reducing gas. The effect of particle size on the reduction rate is not as notable as expected, because of the cracks formed during the reduction. Based on the experimental data as well as the physical condition of sample particles used in this work, the nucleation and growth rate equation best represent the data. The activation energy was determined to be
66 kJ/mole[8].
The aim of this work is studying the reduction of low grade manganese ore pellets by hydrogen.
2-EXPERMENTAL WORK
2-1- Chemical analyses of low grade manganese ore
The low grade of manganese ore used in this work was provided by Sinai ferromanganese Co. The samples of low manganese ore were submitted to chemical and X ray analysis.
The X- Ray analysis is illustrated in Fig.1. From which it is clear that low grade manganese ore mainly consists of pyrolusite, hematite and quartz.
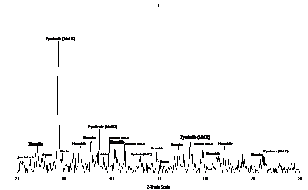
Fig.1. X-ray analysis of low grade of manganese ore
The chemical analysis of low grade manganese is the same like analyses which present in literature [4,9] and is illustrated in Table 1
Constituent | Weight % |
Fe total | 23.2 |
KR2RO | 0.25 |
AlR2ROR3 | 2.3 |
MgO | 0.95 |
CaO | 2.4 |
P | 0.2 |
Mn | 28.6 |
SiOR2 | 15.3 |
NaR2RO | 0.2 |
Table 1, Chemical analysis of Egyptian low grade of manganese ore
2-2-Preperation of Pellets sample

The low grade manganese ore was ground in a vibrating mill to a size less than 75 micro- meters. The ground low grade manganese ore powder was mixed with different percentages of molasses and then 200 gm of mixture was fed to the laboratory disc pelletizer (diameter 400 mm, collar height 100 mm) as in Figure 2 under the following condition: - angle of inclination 52o, disc rotating speed 17 rpm and residence time 30 min. The predetermined amount of water (9%) with molasses was sprayed onto the rolling bed of material in the disc pelletizer. At the end of the tests, a pellet sample was collected and screened to collect the (5-7 mm diameter) fraction. The produced green pellets were dried in air for three days, to ensure the evaporation of water used during the pelletization process. The green and dry pellets
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 341
ISSN 2229-5518
Fig. 2 Disc pelletizer equipment
were then subjected to drop damage resistance and compressive strength tests. The drop damage resistance indicates how often pellet can be dropped from a height of 46 cm before they show perceptible cracks or crumble. Ten pellets (5-7 mm) were individually dropped onto a steel plate. The number of drops is determined for each pellet. The arithmetical average values of the crumbing behavior of the ten pellets yield the drop damage resistance [9-
12]. Ten pellets were compressed between parallel steel plates up to failure to determine the average compressive strength [9-12].
2-3- Reduction process
Reduction of the produced pellets was performed in a thermo balance apparatus shown in Figure (3) [10-
16]. It consists of a vertical furnace, an electronic balance for monitoring the weight change of reacting sample and a temperature controller. The sample is placed in a nickel chrome crucible, which was suspended under the electronic balance by Ni-Cr wire. The furnace temperature was raised to the required temperature 700°C to 950 °C and maintained constant to ± 5°C. The nitrogen flow rate was 0.5 l/min pass through furnace in all the experiments. At initial time air should be removed before each experiment and also after the end of reduction. Then the sample was placed in hot zone and the hydrogen then passed. The weight of the pellets sample was continuously recorded and at the end of the run; the samples were withdrawn from the furnace and put in the desiccators.
The percentage of reduction was calculated according to the following equations:
Percent of reduction of pellets = [(Wo –Wt) x100/ Oxygen mass] (1)
Where:
Wo: the initial mass of low grade of manganese of pellets sample after removal of moisture.
Wt: mass of sample after each time, t.
Oxygen mass: indicates the mass of oxygen percent in low grade of manganese ore in form FeO, Fe2 O3 and manganese oxide.
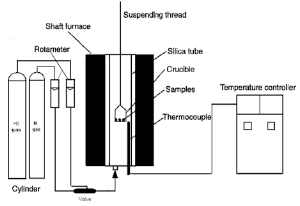
Fig. (3) A schematic diagram of of the reduction apparatus
3-Results and Discussion
3-1- Effect of the amount of molasses added on the quality of the green pellets of low grade of manganese ore
In this experiment, the powder of low manganese ore
with different amount of molasses were pelletized in disc pelletizer with 6% of water (inclination of disc pelletizer =52° and the produced pellets remained in the disc pelletizer for 30 min).
Figures 4 - 5 show the effect of the amount of molasses added to the low grade of manganese ore on the drop damage resistance and compressive strength of the green produced pellets. From these figures, it is clear that both drop damage resistance and compressive strength of green pellets increased as the percentage of molasses increased, this may be due to increase of Vander Waals forces [17-18]. And also this may be due to the molasses more viscous and may be the surface tension of water & gravitational force creates pressure on particles, so they coalesce together and form nuclei which grow in size into ball [19].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 342
ISSN 2229-5518
70
60
50
40
30
20
10
0
0 0.5 1 1.5 2 2.5 3
percentage of binding material added (molasses), %.
compressive strength of the dried produced pellets. From these figures, it is clear that both drop damage resistance and compressive strength increased as the percentage of molasses added increased.
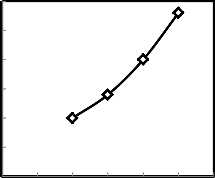
30
25
20
15
10
5
0
Fig.4.Effect of change of molasses addition on the drop damage resistance of green produced pellets
0 0.5 1 1.5 2 2.5 3
Percentage of binding material
added (molasses), %.
0.03
0.025
0.02
0.015
0.01
0.005
0
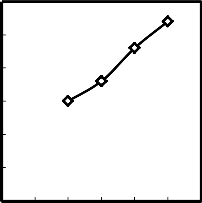
0 0.5 1 1.5 2 2.5 3
Percentage of binding material added (molasses), %.
Fig.6.Effect of change of molasses addition on the drop damage resistance of dried produced pellets
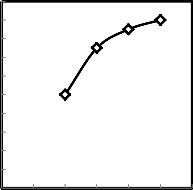
0.2
0.18
0.16
0.14
0.12
0.1
0.08
0.06
0.04
0.02
0
Fig.5. Effect of change of molasses addition on the compressive strength of green produced pellets.
3-2- Effect of the amount of molasses added on the quality of the dried pellets of low grade of manganese ore after 3 day
In this experiment, the dried of low manganese pellets which contains different amount of molasses were subjected to drop damage resistance test and compressive strength test and the results were fitted in Figures (6 – 7). Figures (6 - 7) show the effect of the amount of molasses added to the low grade of manganese ore on the drop damage resistance and
0 0.5 1 1.5 2 2.5 3
Percentage of binding material added (molasses), %.
Fig.7.Effect of change of molasses addition on the compressive strength of dried produced pellets.
3-3- Effect of hydrogen flow rate on the reduction degree
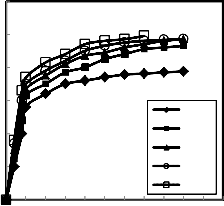
Figure 8 illustrates the relation between the reduction
degree of pellets and hydrogen flow rate when the reduction was done at constant temperature (900oC) and constant weight of the sample. It is clear that as the flow rate of hydrogen increased the reduction
percentage increased, this may be the increase of
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 343
ISSN 2229-5518
flow rate leads to an increase of number of hydrogen mole in the bulk phase, which in turn leads to the raise of hydrogen adsorption and subsequently the rate of reaction increased (20-21) or the increase of flow rate increased the gas diffusion across the boundary layer subsequently the reduced ion increased (15). Also may be the higher flow rate prevailing in the reaction zone which enhances the rate of hydrogen absorption and subsequently the rate of chemical reaction steps increased ( 14).
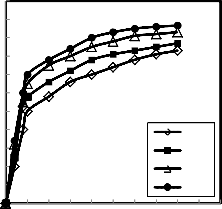
110
100
90
80
120
100
80
60
40
20
0
750ºC
800 ºC
850 ºC
900 ºC
950ºC
0 5 10 15 20 25 30 35 40 45 50 55
Time of reduction , min.
. 70
60
50
40
30
20
10
0
1/2 L
1 L
1.5 L
2 L
0 5 10 15 20 25 30 35 40 45 50
Time of reduction, min.
Fig.9.Effect of change the reduction temperature on the reduction degree at different time of reduction and at constant hydrogen flow rate =1.5 liter /min.
3-5-Kinetics reduction for pellets of low grade manganese ores
Kinetic studies for estimation of apparent activation
energies were carried out for pellets of low grade manganese ore at different temperatures from 750˚C up to 950˚C for different time intervals in the range of 5 - 60 minutes by using chemical control Equation
Fig.8.Effect of change hydrogen flow rate on the reduction degree at different time of reduction and at constant temperature (900oC).
3-4-Effect of the reduction temperature on the reduction degree
The reduction was carried out at different
temperatures ranging from 750 up to 950 ºC, where the weight of the pellets were constant and the hydrogen flow rate =1.5 liter /min. The results of the investigation are shown in Figure 9, for the pellets binding by 2% molasses. It is clear that the increase of temperature favors the reduction rate and degree of reduction. The increase of reduction percentage with rise of temperature may be due to the increase of number of reacting moles having excess of energy which leads to the increase of reduction rate (20, 22-
24). Also the raise of temperature leads to an increase of the rate of mass transfer of the diffusion and rate of desorption (14-15, 21-23).
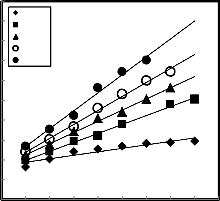
[25] :- - ln (1-R) = kt (2) Where:- R is fractional reduction, t is time of reduction and k is the rate constant.
Figure 10, illustrates the relation between – ln (1-R) against time of reduction for different reduction temperature. From which it is clear that the relationship is represented by straight line.
The natural logarithms were used according to the
Arrhenius equation to calculate the activation energies of reduction reaction by using the calculated rate constant k.
k = k0 exp E /RT (3)
ln k = ln k 0 - E /RT (4) Where k0 is the coefficient; E is the apparent reduction activation energy; R is the universal gas constant [8.314 × 10−3 kJ/mole ∙K)]; T is the absolute temperature. The relationships between the natural logarithm of reduction rate constant and the reciprocal of absolute temperature for low grade of manganese ore pellets are shown in Figure 11, from which it is clear that the reduction of pellets has
activation energy 79.78 kJ /mole.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 344
ISSN 2229-5518
5
4.5
4
750ºC
800ºC
850ºC
y = 0.0702x + 0.8418
3.5
900ºC
950ºC
y = 0.0905x + 0.8799
R² = 0.9881
R² = 0.994
3
2.5
2
1.5
1
0.5
0
y = 0.0563x + 0.8444
R² = 0.9963
y = 0.0452x + 0.7618
R² = 0.9952
y = 0.0174x + 0.8561
R² = 0.8906
0 5 10 15 20 25 30 35 40 45
time of reduction , min.
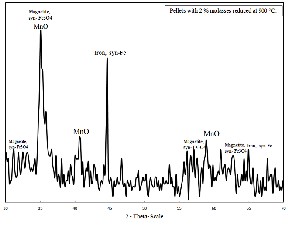
Fig.12. XRD analysis of reduced manganese ore pellets with 2 % molasses at 900°C.
Fig.10. Relationship between time of reduction and
-ln (1-R) at different reduction temperatures
0
-0.5
-1
-1.5
4- Conclusions
Manganese plays an important role in several industrial applications. The utilization of the low grade manganese ore has become necessary. There are several physicochemical differences among the components in manganese ores, which can be used for the enrichment of manganese. In particular, the
-2
-2.5
-3
-3.5
-4
-4.5
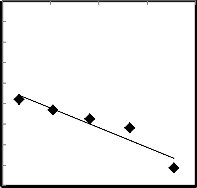
y = -9596.2x + 5.5542
R² = 0.896
abundant low-grade manganese ores, which contain iron oxide, may be upgraded by pre-reduction and magnetic separation. The reduction of Egyptian low grade of manganese ore by hydrogen in temperature indicated that:-
1-The reduction rates increased with increasing
temperature of the reduction.
0.0008 0.00085 0.0009 0.00095 0.001
1/T (k-1)
Fig.11. The relation between the reciprocal of absolute temperature 1/T and lnK (Arrhenius plot for reduction reaction) for model - ln (1-R) = kt
3-5- X-Ray of the reduced pellets
The X- ray analysis of low manganese ore pellets with 2% molasses and reduced at 900 °C at hydrogen flow rate =1.5 liter /min. is illustrated in figure (12). From which it is clear that the main phases in the sample consist of Iron (syn. Fe) , Magnetite (syn.Fe3O4) and Manganese oxide (MnO).
2-Increase hydrogen flow rate at constant temperature of reduction leads to increase rate of reduction.
3-The reduction of low grade manganese ore controlled by chemical reaction control and the activation energy = 79.78 kJ/mole.
5- References
1. Sahoo, R.N., Naik, P.K.and Das, S.C., “Leaching of manganese ore using oxalic acid as reductant in sulphuric acid solution”, Hydrometallurgy 62, 157–
163, 2001.
2.Hazek M.N. El, Lasheen T.A. and Helal, A.S., “Reductive leaching of manganese from low grade Sinai ore in HCl using H2 O2 as reductant”, Hydrometallurgy 84,187–191, 2006.
3.Haifeng Su, Yanxuan Wen, Fan Wang, Yingyun
Sun and Zhangfa Tong, “Reductive leaching of
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 345
ISSN 2229-5518
manganese from low-grade manganese ore in H2 SO4
using cane molasses as reductant”, Hydrometallurgy
93, 136–139, 2008.
4-Hala H. Abd El-Gawad, Ahmed M. M., El-Hussiny N. A., Shalabi M. E. H., “Reduction of low grade Egyptian manganese ore via hydrogen at 800˚C -
950˚C”, Open Access Library Journal, l, 1: e427. July
2014.
5- Yubo Gao, “Pre-reduction and magnetic separation of low grade manganese ore”, Master of Science Department of Metallurgical Engineering University of Utah, August 2011.
6- Ismail Seçkin Çardakli, M.Sc., “Production of high carbon ferromanganese from a manganese ore located in Erzincan”, The graduate school of natural and applied sciences of Middle East technical university, September 2010.
7-V. Kivinen, H. Krogerus and J. Daavittila, “Upgrading of Mn / Fe ratio of low grade manganese ore for ferromanganese production, The Twelfth International Ferroalloys Congress Sustainable Future ,Helsinki, Finland, 467-476, June 6 – 9, 2010.
8- El-Hussiny N.A.. Hala H Abd El-Gawad , Marwa M Ahmed , Shalalabi M.E.H., “Reduction of low grade Egyptian maganese ore by carbon of coke breeze in the briqutte form”, Journal of Multidisciplinary Engineering Science and Technology (JMESTI) Vol.1. No.1, pp. 77-82, January, 2015.
9- K. Mayer, "Pelletization of Iron Ores", Springer- Verlag Berlin Heidelberg, (1980).
10-El-Hussiny N.A., Shalabi M.E.H.,“A self- reduced intermediate product from iron and steel plant waste material using a briquetting process” , Powder Technology, 205, 217-223, 2011
11-Naglaa Ahmed El-Hussiny ,Inass Ashraf Nafeaa
,Mohamed Gamal Khalifa , Sayed Thabt.Abdel- Rahim,Mohamed, El-Menshawi Hussein.Shalabi,” Sintering of the briquette Egyptian iron ore with lime and reduction of it via hydrogen”, International Journal of Scientific & Engineering Research, Volume 6, Issue 2, 1318-1324, February-2015.
12-Nagwa Mohamed Hashem , Bahaa Ahmed Salah
, Naglaa Ahmed El-hussiny , Said Anwar Sayed , Mohamed Gamal Khalifa . Mohamed El-Menshawi Hussein Shalabi, , “Reduction kinetics of Egyptian iron ore by non coking coal”, International Journal of Scientific & Engineering Research, Volume 6, Issue
3, 846-846, March-2015.
13-El-Hussiny N.A, Abdel-Khalek N.A, Morsi M.B, Mohamed O.A, Shalabi M.E.H. Baeka A.M. , 1996, “Influence of water amount added on the sintering process of Egyptian iron ore”, Gornictwo, Vol. 231, PP. 93-115.1996
14- Sayed S. A., Khalifa M.G., El-Faramawy E.S.R., Shalabi M.E.H., “Reductions kinetic of El-Baharia iron ore in a static bed.”, Gospodarka Surowcami Mineranymi, Vol.17 - special issue, 241-245, 2001.
15- Sayed S.A., Khalifa G.M., El-Faramawy E.S.R.,
Shalabi M.E.H., “Kinetic reduction of low manganese iron ore by hydrogen”, Egypt. J. Chem,
45, No. 1, pp.47-66, 2002.
16- Gaballah N. M., Zikry A. F. , Khalifa M. G. , Farag A. B., El-Hussiny N. A., Shalabi M. E. H., “Kinetic reduction of mill scale via hydrogen”, Science of Sintering, 46, 107-116, 2014.
17--Mangena S.J., and Du Cann V.M., “Binder less
briquetting of some selected South African prim coking, Blend coking and Weathered bituminous coals and the effect of Coal properties on binder less briquetting”, International Journal of Coal Geology
71, (303-312), 2007.
18--Mohamed F.M., Ahmed Y.M.Z., and Shalabi M.E.H., “Briquetting of waste manganese ore sinter fine using different binding materials”, Environmental issues and waste management in energy and mineral production SWEMP, 567-573,
2004.
19 -Asima Priyadarsini , Itishree Mishra , “Reduction kinetics of iron ore pellets and , the effect of binders, A thesis submitted in partial fulfillment of the requirements for the degree of Bachelor of Technology In Metallurgical and Materials Engineering, Department of Metallurgical and Materials Engineering National Institute of Technology Rourkela, 2007.
20- Shalabi M. E., “The kinetics of reduction of Baharia iron ores with hydrogen on static bed,” M.Sc., El-Tabbin Metallurgical Institute for Higher Studies, 1973.
21- Abdel Gawad H.H., Hussiny N.A. , Wassf M.A. , Khalifa M.G. , Iskander B.A. , Shalabi M.E.H. , “Briquetting of Egyptian Ilmenite ore with different organic binder and reduced its in hydrogen in temperature range 800 – 1200 ºC”, Science of Sintering, 46 , 205-216, 2014.
22- Gaballah N. M., Zikry A. F., Khalifa M. G.,
Farag A. B., El-Hussiny N. A. , Shalabi M. E. H.,
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 346
ISSN 2229-5518
“Production of iron from mill scale industrial waste via hydrogen , Open Journal of Inorganic Non- Metallic Materials, 3, 23-28, 2013.
23- El-Hussiny, N.A., Abdel-Khalek, N.A., Morsi, M.B., Mohamed, O.A., Shalabi, M.E.H. and Baeka A.M. “Influence of water amount added on the sintering process of Egyptian iron ore. Gornictwo,
231, 93-115,1996.
24-Naglaa Ahmed El-Hussiny, Hassan Hussein Abdul-Wahab, Mohamed Mahmoud Ali, Abdud- Lattif Abdel-Motagally Omar, Mohamed El- Menshawi Hussien Shalabi , Mohamed Reda Moharm , “Effect of grinding time of mill scale on the physicochemical properties of produced briquettes and its reduction via hydrogen”, International Journal of Scientific & Engineering Research, Volume 6, Issue 1, pp. 1641-1659, January-2015.
25- Sinha K M K, Sharma T., Haldar D.D., “Reduction of Iron Ore with Non Coking Coal”, International Journal of Engineering and Advanced Technology (IJEAT) ISSN: 2249 – 8958, Volume-3,
Issue-3, 30-33, February 2014.
IJSER © 2015 http://www.ijser.org