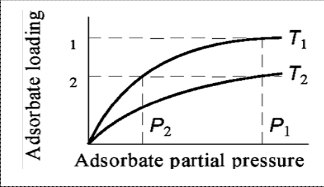
frequently applied process cycles for gas separation. The basic difference between the two processes is shown schematically in Figure (1).
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015
ISSN 2229-5518
597
Abstract- An adsorption (PSA) unit consist of two – tubes columns pressure swing, (6cm diameter and 70cm bed length) and a dryer part (12cm diameter and 27cm) filling with activated alumina (Al2O3) have been constructed to study the separation of oxygen from air using commercial 5A zeolite under the effect of adsorption pressure (1 to 6 bar), adsorption time (20s), product flow rate (1 liter/min) on the product oxygen purity. For the case of 2-column, 4-step operation, the results show that an optimum concentration product of oxygen was 76.9%purity, at the adsorption pressure 4bar, Temp 17.4oC.
Key Words-Pressure swing adsorption (PSA), Oxygen production using Zeolite 5A, Air separation, Equilibrium adsorption, Oxygen concentrator unit, Activated alumina , Adsorption pressure.
--------------------------------------------------------------------------------------------------------------------------------
dsorption processes are often identified by their method of regeneration. Temperature-swing adsorption (TSA) and pressure swing adsorption (PSA) are the most
frequently applied process cycles for gas separation. The basic difference between the two processes is shown schematically in Figure (1).
Fig-1: Temperature- swing and pressure-swing cycles.
Because changes in pressure can be brought about more rapidly than changes in temperature, pressure-swing regeneration can be used with shorter cycle-times than was possible with temperature-swing. This, in turn, allows smaller beds to be used and consequently a smaller inventory of adsorbent is needed in the system [1, 2 and 3].
Atypical total cycle time for the PSA process is between one to several minutes [4], compared to the TSA process cycle time of hours.
————————————————
Department of Fuel and Energy, Technical College-Kirkuk, Iraq
Pressure swing adsorption (PSA) processes, first suggested by Skarstrom in the form of a ‘heatless drier’ have found widespread application in hydrogen purification and air separation, as well as in air drying [5,6 and 7].The PSA process could be described briefly as follows:
Two columns operate semi-continuously in four stages to supply the desired purity and flow rate of oxygen. Adsorbents are selectively chosen to provide for the most beneficial design. The PSA stage operation is described in Figures 2-5[8, 9] as following;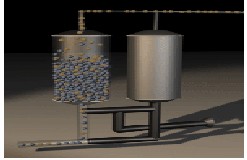
-Stage 1: Compressed air is fed into the first bed. Nitrogen molecules are trapped, while oxygen is allowed to flow through Fig (2).
Fig-2: PSA Stage1
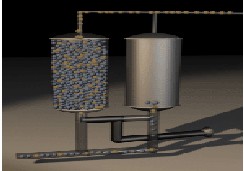
-Stage 2: When the adsorbent in the first bed becomes saturated with nitrogen, the air flow feed is directed into the second bed Fig (3).
Fig-3: PSA Stage2
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015
ISSN 2229-5518
598
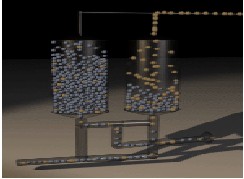
-Stage 3: The adsorbent adsorbs nitrogen in the second bed. The first bed is depressurized allowing nitrogen to be purged out of the system and released to the atmosphere Fig (4).
Fig-4: PSA Stage3
-Stage 4: The process starts over. Compressed air is once again fed into the first bed. The second bed is depressurized releasing argon and nitrogen molecules to the atmosphere. The process is repeated continuously producing a constant flow of purified oxygen Fig (5).
Fig-5: PSA Stage4
A very important improvement was the introduction of the pressure equalization step. [10,11 and 12].The pressure equalization, using a product-enriched current, leads to a significant economy in energy consumption, since less mechanical energy is required to re-pressurize the column at low pressure, after the purge stage. The product recovery is, in this way, also increased because less feed gas is necessary to re-pressurize the column. [13].
Oxygen production (purity below 95%) from air, using nitrogen selective zeolites of type (5A) or X (13X-NaX, LiX, or LiLSX), by means of pressure swing adsorption (PSA) processes has noticeably increased in the past decade. However, the concentration of the product is limited to 95% oxygen, because of the presence of argon in air, since these adsorbents present similar adsorption capacities for oxygen and argon. [14].
Zeolites have the unique ability to adsorb N2 more strongly than O2 (i.e., by a factor of 2 or more in terms of pure component adsorption amounts). The main reason for this is the interaction between the quadruple moment of N2 and the cation that is attached to the zeolite framework. [15].
Nitrogen and oxygen are, respectively, the second and third largest man-made commodity chemicals today. Since
1920s, N2 and O2 have been produced by cryogenic distillation of air. Since 1980s, adsorption (i.e., pressure swing adsorption or PSA) has been adopted rapidly and increasingly for air separation. Approximately 20% of air separation is presently
accomplished by PSA. [15].
Commercial PSA technology can be used to produce an oxygen-enriched stream containing 80-95% oxygen which
finds application in biological wastewater treatment, medical use, enhanced combustion in furnaces and cupolas, etc.[4].
Currently, design of PSA systems relies heavily on experimental data for the system of interest. Experiments are conducted over a wide variety of process condition (pressure, purge-to-feed rates, steps times, feed and product flow rate, etc.) for specified adsorbent and cycle.
The aims of the present work are:
1. The objective of this project is to determine whether or not it is feasible to build an oxygen supply device using 5A zeolites to separate oxygen from air, using two bed columns, packed with commercial 5A zeolite and its properties shown in table(1)[16].
2. Studying the effects of adsorption pressure on the performance of PSA unit, using two columns 4-steps equalization modification cyclic operations. The Performance is characterized by O2 product purity, maximum operating pressure 6 bar.
The experimental work includes two parts, one for air drying and another for oxygen separation from air using zeolite type
5A to produce pure oxygen. All the runs are conducted at ambient temperature.
Devices;
Air separation by pressure swing adsorption to produce pure oxygen performed using two columns 4-steps equalization modification cyclic operations.
The equipment used in the present work consists of:
1- Two-tubes Adsorption columns of Aluminum type (a) of
6cm diameter and 70cm length filled with zeolite type 5A.
TABLE 1
5A Zeolite properties.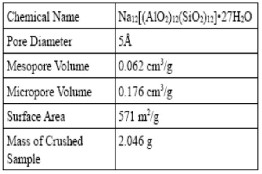
2- Product flow meter (flow rate of 0.1 to 1 liter/min, AFR2000, XCPC2000).
3- The drying unit is composed of one galvanized steel columns, which are packed with activated alumina
(2.395Kg) with properties shown in table (2).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015
ISSN 2229-5518
599
Table (2) activated alumina properties
TABLE 3
Oxygen Meter Model: DO-5510HA.
Super large LCD display with
4- The length of column (L) is 27 cm and its diameter (D) is
12cm Fig (6).
Fig-6: Drying unit.
5- Digital Oxygen meter with a polar graphic type probe with an incorporated temperature sensor which serves for precise Dissolved Oxygen (DO) and temperature measurement. Model: DO-5510HA with specifications shown in table (3), Fig (7).

Fig-7: Oxygen meter unit.
6- Purge flow meters (Maximum flow rate 10L/min, Type
YR-88, manufacture in china).
7- Pressure regulator (1 to 10 bars).
8- Solenoid valves on the feed line, (Model 2W-200-20, 3/4" in size, 220V, 50Hz, 28VA, Temp (-5ºC-80ºC), H.A.K
pneumatic).
9- Equalization valves (1/8" in size, 220V, 50Hz, Type122k232, 30bar, Lucifer Switzerland.).
10- Pressure gauges (1-8 bar, china manufactured).
11- Control panel consist of four steps to operate the unit automatically Fig(8), which is working as follow;
A-The first 20 seconds, V1 (2 and 4 valve) is open. The remaining valves are closed.
B-For 10 seconds, V2 (5 and 6 valves) are open. The remaining valves are closed.
C-For 20 seconds, V3 (1 and valve 3) open. The remaining valves are closed.
D-For 10 seconds, (5 and 6 valves) open. The
remaining valves are closed.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015
ISSN 2229-5518
600
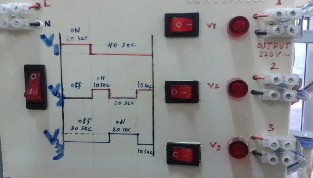
Fig-8: control panel 4 steps unit.
9- Each column contains 1.430Kg of zeolite 5A.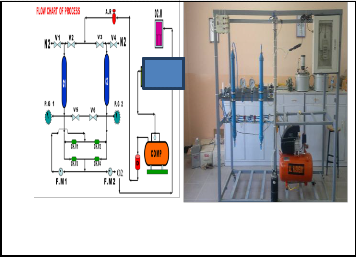
10- Flow diagram and picture of PSA unit Fig (9).
Air
C | F.M | CH.V | P.G | V | A.R | D | O2.M | COMP |
colu mn | Flow meter | Check valve | Pressure gauge | Valv e | Air regulator | Dryer | Oxygen meter | Compr essor |
Fig-9: Schematic diagram and picture of PSA unit.
Pressure swing adsorption (PSA) is a technology used to separate some gas species from a mixture of gases under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at near-ambient temperatures and differs significantly from cryogenic distillation techniques of gas separation. Specific adsorptive materials (e.g., zeolites, activated carbon, molecular sieves, etc.) are used as a trap, preferentially adsorbing the target gas species at high pressure. The process then swings to low pressure to desorb the adsorbed material.
Effect of higher operation pressure studied on Oxygen purity in laboratory scale PSA unit from 1 to 6 bar using Zeolite 5A. In this system binary mixture (N2/O2) (79/21 vol %) used as a feed stream as following:
1- Air compressor started until the tank pressure reached the desired pressure (4bar for O2).
2- Operate the control panel.
3- Then the air enters to dryer to drying from moisture
and cleans it from impurities.
4- Regulate the air by the air regulator to the require pressure.
5- The air enters to the column1 (c1) from the top, the zeolite adsorbs nitrogen and the other gases and oxygen rich gas flow from the bottom.
6- The oxygen from column1 divided into two streams.
7- The second stream goes to the valve 5 to equivalent pressure between the column 1 and column 2.
8- the third stream goes to the check valve 1 that is reversal direction of it flow, therefore goes to the check valve 3 that is the same direction of its flow and
allow it to flow across it.
9- From check valve 3 divided into two streams.
10- The first stream goes to the flow meter2, and the
product oxygen goes to oxygen meter to measure its
concentration and temperature.
11- The second stream goes to the flow meter 1 then recycled.
12- The recycle is entering from the bottom of column 1 to desorb the nitrogen from the zeolite to clean it, and to be ready to another adsorption process of oxygen.
13- The nitrogen out from the top of the column 1, and valve 1 is open then nitrogen out from it.
14- The processes from step 5 to 14 is the same process in the column 2,but when the first column is adsorbed nitrogen and product oxygen the second column is
desorbed nitrogen from zeolite by the oxygen recycle.
15- And when the second column is adsorbed nitrogen and product oxygen the first column is desorbed nitrogen from zeolite by the oxygen recycle.
Oxygen concentrators operate on the principle of rapid pressure swing adsorption of atmospheric nitrogen onto zeolite minerals and then venting the nitrogen. This type of adsorption system is therefore functionally a nitrogen scrubber leaving the other atmospheric gasses to pass through. This leaves oxygen as the primary gas remaining. PSA technology is a reliable and economical technique for small to mid-scale oxygen generation, with cryogenic separation more suitable at higher volumes and external delivery generally more suitable for small volumes.
The separation of mixtures of fluids using zeolitic as selective adsorbents may be accomplished in three fundamentally different modes;
1-When the physical size and or shape of the molecules of the fluid materials to be separated are sufficiently different and a molecular sieve having a suitable pore size is available a separation is possible based on the acceptance-exclusion phenomenon.
2-When the physical size and or shape of the molecules to be separated are different and a molecular sieve is available having
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015
ISSN 2229-5518
601
a pore opening through which the larger molecules pass only with difficulty, a separation is possible which is kinetically controlled. Some such separations are inverse to the separation effected when the molecules all can freely enter the pore system.

3-When the physical size and or shape of the molecules to be separated are not sufficiently different to either be separated by the acceptance-exclusion phenomenon or the kinetically controlled situation but all freely enter the pore system the separation depends on the forces of adsorption between the molecular sieve and the different molecules.
Pressure swing adsorption processes rely on the fact that under high pressure, gases tend to be attracted to solid surfaces, or "adsorbed". The higher the pressure, the more gas is adsorbed; when the pressure is reduced, the gas is released, or desorbed. PSA processes can be used to separate gases in a mixture because different gases tend to be attracted to different solid surfaces more or less strongly. If a gas mixture such as air, for example, is passed under pressure through a vessel containing an adsorbent bed of zeolite that attracts nitrogen more strongly than it does oxygen, part or all of the nitrogen will stay in the bed, and the gas coming out of the vessel will be enriched in oxygen. When the bed reaches the end of its capacity to adsorb nitrogen, it can be regenerated by reducing the pressure, thereby releasing the adsorbed nitrogen. It is then ready for another cycle of producing oxygen enriched air.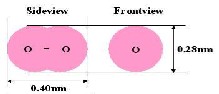
Fig-10: View of oxygen atomic.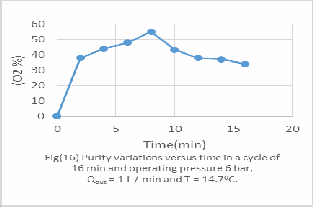
At high pressure, the porous zeolite adsorbs large quantities of nitrogen, due to its large surface area and chemical character. After the oxygen and other free components are collected the pressure drops which allows nitrogen to desorb.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015
ISSN 2229-5518
602
- Small: Estimated .2m x 2m x 1m
Thus from the above information, it can be see that a competitive / light weight portable oxygen concentrator with
76.9% oxygen can be produced. It is useful for using in welding machine and it is small enough to take on an airplane. It is a good idea to study the effect of ozone on the oxygen and nitrogen production.
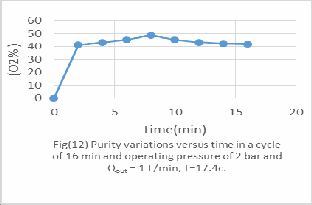
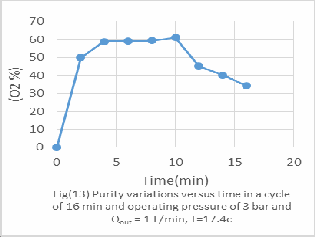
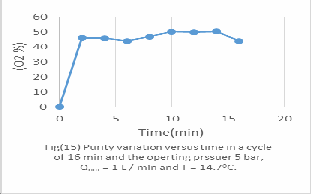
All figs (11-16) represent the PSA performance under the same feed rate from 1 to 6 bar and it's evaluated experimentally as following;
For Oxygen
-For 1 bar the optimum Oxygen concentration 38%vol after 14 min operation.
-For 2 bar the optimum Oxygen concentration 58%vol after 8 min operation.
-For 3 bar the optimum Oxygen concentration 46%vol after 10 min operation.
-For 5 bar the optimum Oxygen concentration 55%vol after 8 min operation.
-For 6 bar the optimum Oxygen concentration 50.3%vol after
14min operation.
Purity is increased with increasing of purge and after reaching to a peak, decreasing. As we know, purging the adsorption bed, help the adsorbent regeneration and increase its capability, but increasing the amount of this stream more than its sufficiency causes the adsorption of oxygen on the adsorbent because of its high vapor pressure in the column and the efficiency of adsorbent will be decreased.
With increase in the product amount, the peak of purity is coming down. Large amount of product stream is because of large amount of feed stream. The large amount of feed stream, the low efficiency separating of feed stream, because of large volume of gas in the adsorption bed at the same time.
As we see from fig (11), in short cycle time, high purity will be achieved. Optimum oxygen concentration 76.9%vol after 4 min operation time. Because there is not enough time for oxygen adsorption instead of nitrogen. This laboratory unit PSA it is suitable to operation under 4 bar from the practical results calculated.
The following goals were met with the portable oxygen concentrator from the initial estimates;
- Purity: 76.9% oxygen, (from the first stage).
- Cost: $4200 (under $5000).
- Weight: 75kg.
References:
[1] Moghadazadeh Zahra, TowfighiJafar, and MofarahiMasoud, "Study of a four-Bed Pressure Swing Adsorption for Oxygen Separation from air", In. J. Chem. Bio. Eng. 1.3 (2008).
[2] Masoud Mofarahi, EhsanJavadiShokroo, "COMPARISION OF TWO PRESSURE ADSORPTION PROCESS FOR AIR
SEPARATION UZING ZEOLITE 5A AND ZEOLIT
13X".Petrolum & Coal 55(3)216-225, (2013).
[3] Kirk-Othmer, Encyclopedia of Chemical Technology, volume 1,4th edition,
John-Wiley & Sons (1991-1998).
[4] Sircar, S., "Air Fractionation by Adsorption,
Separation Science &Technology", 23(14 & 15), pp.
2379-2396, (1988).
[5] Skarstrom, C. W., "Use of adsorption phenomena in automatic plant-type gas analyzers". Ann. N.Y. Acad. Sci. 72, 751, (1959).
[6] Skarstrom, C. W., U.S. patent 2,444,627 (to ESSO
Research and Engng Co.), (1960).
[7] Skarstrom, C. W., "Heatless fractionation of gases over solid adsorbents", in Recent Developments in Separation Science, Vol. 2. CRC Press, Cleveland, Ohio, 1972.2, 95-106 (1972).
[8] Ruthven, D. M.; Farooq, S.; Knaebel, K. S. "Pressure Swing
Adsorption". VCH Publishers: New York, (1994).
[9] Pressure Swing Adsorption Technology. Retrieved March 15, (2007), from Oxygen Generating System Intl. Web site:
http: www.ogsi.com/pressure_swing_adsorption_technology.php. (2007).
[10] Pramuk, F. S., Hoke, R. C., &Skarstrom, C. W., "Pressure equalization depressurizing in heatless adsorption". US Patent
No. 3 142 547, (1964).
[11] Berlin, N. H., “Method for providing an oxygen-enriched
environment”. US Patent No. 3 280 536, (1966).
[12] Wagner, J. L.," Selective adsorption process". US Patent
No.3430418 (1969).
[13] Cruz, P., Santos, J. C., Magalhaes, F. D. and Mendes, A.," Cyclic adsorption separation processes: analysis strategy and optimization procedure", Chem. Eng. Sci., 58, 3143 – 3158, (2003).
[14] Santos J.C., Cruz P, Regala T., Magalhaes F.D., and Mendes A., "High- Purity Oxygen Production by Pressure Swing
Adsorption", IndEngchem 46, pp 591-599, (2007).
[15] Rege, S.U., and Yang, R.T.," Limits for Air Separation by
Adsorption with LiX Zeolite", Ind. Eng. Chem. Res., 36, 5358-
5365, (1997).
[16] Anant J. Songsde and R. V. Prajapati.,”Pressure swing adsorption a Cleaner techniques to reduce emission”, IJSRD, vol.1, Issue 3, (2013).
IJSER © 2015 http://www.ijser.org