
surface ofthe gel electrolyte (figure b) and the average size of isolated NH4SCN particles is 7.71μm .
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 1719
ISSN 2229-5518
OPTICAL STUDY OF PVA BASED GEL ELECTROLYTE
1Shital N. Bhad, 2V.S.Sangawar
1Student, Polymer Research Laboratory, P.G. Department of Physics, G.V.I.S.H, Amravati, Maharashtra, India, bhad_sheetal@yahoo.co.in
2Professor, Polymer Research Laboratory, P.G. Department of Physics, G.V.I.S.H, Amravati, Maharashtra, India, vijayasangawar@yahoo.co.in
Abstract—An attempt has been made to prepare polyvinyl alcohol (PVA) based gel electrolyte in ammonium thiocyanate(NH4SCN) solution and to characterised it by SEM, FTIR & UV-absorption study. SEM revealed in the 0.5gm and 2.5gm PVA+NH4SCN gel electrolyte, PVA acts as the backbone and NH4SCN particle are distributed randomly both on the surface and in the internal structure of PVA backbone. FTIR result indicates the presence of active interaction between PVA and NH4SCN in these gel electrolytes. From UV- study, the direct band gap is calculated ,and it found that it decreases from 5.57 ev to 3.17ev as PVA concentration increases in gel electrolyte.
—————————— ——————————
During the last decades there was a lot of effort done on the electrochemical preparation of polymer gel electrolytes. Polymer electrolyte have become material of great importance for use in different electrochemical devices due to their unique characteristics such as easy mouldabality, good electrode –electrolyte contact and light weight.[1]
Over the year’s polymer gel electrolyte have been given impetus in electrochemical devices due to certain distinct advantages over solid polymer electrolytes, particularly ionic conductivity that approaches to that of liquid electrolyte. [2]
In recent time, development of different synthesis techniques of gel electrolyte such as soft chemical and sol gel method have led to the engineering materials [3].These gel electrolyte possesses soft morphology .Polyvinyl alcohol (PVA) in solution can form a gel under proper conditions. Preparation and properties of PVA gel have been extensively studied in the past. (4-8).PVA may be used for making different kinds of articles such as laundry bags, packing items and various purposes including for the preparation of gel and the like (9,10)PVA seems to be a very attractive material for preparation of gel. PVA has both excellent mechanical property and chemical stability [11]. PVA is a basic polymer which has good film forming capacity, thermal stability and mechanical property [12-13] .The optical properties are aimed at achieving better reflection, antireflection, interference and polarization properties.[14]
In the present investigation, an attempt has been made to synthesize PVA based gel electrolyte with ammonium salt and study of its optical properties.
In the present study polyvinylalcohol, ammonium thiocyanate (NH4SCN) and aprotic solvent dimethylsulfoxide (DMSO) are of AR grade use for synthesis of gel.
2.2 Synthesis
For the synthesis of PVA gel 30 ml electrolyte of
0.2 M solution of NH4SCN + DMSO was prepared. PVA
was added in the electrolyte with constant stirring at 70
0C temperature and was maintained for one hour. Then it
was allowedto cool to room temperature so as to form the
gel. PVA hasbeen added in the electrolyte in a different
amount 0.5, 1, 1.5, 2, 2.5 by weight to form the gel of
various concentrations.
The surface morphology of PAV+NH4SCN gel electrolyte studied by scanning electron micrograph is shown in fig.1.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 1720
ISSN 2229-5518

surface ofthe gel electrolyte (figure b) and the average size of isolated NH4SCN particles is 7.71μm .
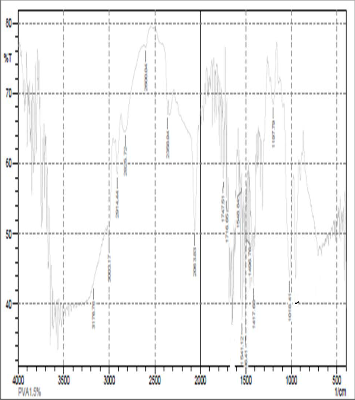
Fig – 1 (a) SEM image of 0.5gm PVA+ NH4SCNgel electrolyte. 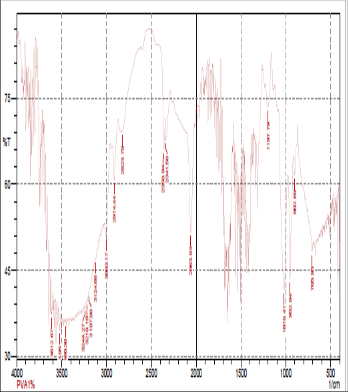
 Fig: 2(a) FTIR of 1gm of PVA
Fig: 2(a) FTIR of 1gm of PVA
Fig – 1 (b) SEM image of 2.5gm PVA + NH4SCN gel electrolyte.
Figure 1(a) and (b) show the SEM images of the surface and the cross-section ofthe 0.5gm and 2.5 gm PVA
+NH4SCN gel electrolyte respectively. Both figures revealed that in the PVA+ NH4SCN gel electrolyte, PVA act as thebackbone and NH4SCN particles were distributed both on the surface and in theinternal structure of PVA backbone. These NH4SCN particles performed as thechannels for proton conduction through the gel. However, it could be seen thatthe NH4SCN particles were not homogeneously distributed among the gel.Aggregations of NH4SCN particles observed at the
Fig: 2(b) FTIR of 1.5gm of PVA
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 1721
ISSN 2229-5518
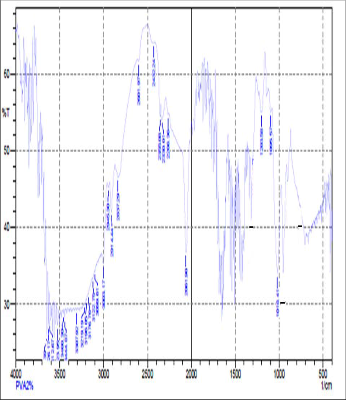
Fig: 2(c) FTIR of 2gm of PVA
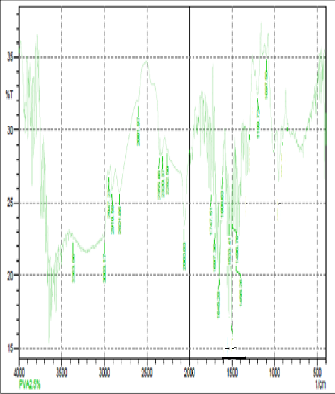
Fig: 2(d) FTIR of 2.5gm of PVA
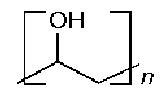
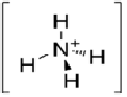
Polyvinyl Alcohol Ammonium thiyocynate
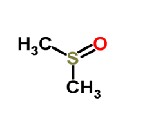
Dimethylsulfoxid
Above Molecular Structure of polyvinyl Alcohol, Ammonium thiyocynate and
FTIR spectroscopy was used to understand the aspect of hydrogen bonding in PVA+NH4SCN+DMSO sample. In fact FTIR spectral data support the phenomenon of stabilization [15] ofthe associative structure though intermolecular H bonding in PVA+NH4SCN. The FTIR spectra for PVA+NH4SCN show absorption due to OH-stretching vibration at 3003 cm-1.The presence of methylene group in PVA can be seen from the 2814cm-1 and 2914-44cm-1.The peak observed at 3176 cm-1indicates the stretching vibration of NH4+ and was found to be disappeared with increase in concentration of PVA. OH-stretching vibration peak shifted towered the lower frequency when compared to pure PVA. This result suggested that hydrogen bonding becomes weaker in cross linked PVA compared to pure PVA because of reduction in number of OH group [16].The peak observed at 1018cm-1indicates the interaction of dimethylsulfoxid with salt. These changes indicated that intermolecular interaction between NH4SCN molecules , molecule of polyvinyl alcohol and DMSO.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 1722
ISSN 2229-5518
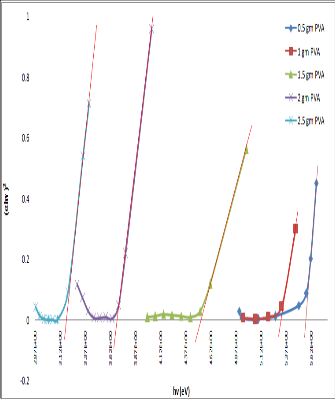
Fig :3 The direct band gap energy of different concentration of PVA
The energy band gap of PVA gel was determined from optical absorption measurement carried out on UV-1700
PC spectrometer. Fig shows the relation between (αhν)2
versus hν plot according to equation
αhν =β(hν- Eopt) m,
where m=2.The direct optical energy gap can be obtained from the intercept of the resulting straight line with the energy axis at (αhν)2=0. The optical energy gap 5.57ev,
5.27ev, 4.52ev, 3.69ev, 3.17ev was found at 0.5gm, 1gm,
1.5gm, 2gm, 2.5gm concentration of PVA respectively.
The optical energy gap decreases from 5.57ev to3.17ev as
concentration of PVA increases. A decrease in the energy band gap occurs in the most cases. There is a strong interaction between a polymer molecule and solvent called as on solvent interaction which enhance optical response of the polymer it may be further noted that the large terminal ends of the polymer are responsible for solute solvent interaction in which the solute is polymer. As concentration of polymer increases in the solution the magnitude of solute-solvent and solute solute interaction changes which results in the structural change in polymer solution.[17][18]The high value may be attributed to better quantum confinement effect of NH4SCN reported here. [19] Our results are good agreement with [20-22].
Polymer gel electrolyte of PVA+NH4SCN+DMSO has been successfully synthesized by freezing and thawing process. SEM shows the aggregation of NH4SCN practical observed at the surface of gel electrolyte. FTIR revealed that the presence of active interaction between PVA and NH4SCN in these gel electrolytes. The optical energy gap can be controlled by adding required amount of PVA in ammonium thiocyanate electrolyte.
[1]. S.N Bhad, V.S Sangawar, Chem Sci Tran. 1(3):653-657,2012.
[2]. N. Chand, , N. Rai ,T.S. Natarajan and S.L Agrawal, Fibers and Polymers,
12(4):438-443 , 2011.
[3]. Gopalakrishanan J.Ghem.Mater. 7: 1265 ,1995.
[4]. N.A Peppas, E. W. Merrill, J.Polym. Sci., Polym.Chem, Edn .:14:441, 1976. [5]. S .Matsuzawa, K .Yamaura, H .Kobayashi, Colloid. PolymSci .25:1147, 1981. [6]. M. Watase, K Nishinari, Macromol. Chem., 186:1081, 1985.
[7]. M. Komastu, T. Inoue , K . Miyasaka J. Polym . Sci., Polym Phys. Edn.;
24:303, 1986.
[8]. H .Toshihiro, M .Hidetoshi, S. Takash, H. Sadao, J.Appl. Polym. Sci.
45:1849, 1992 .
[9]. C.A.Finch, polyvinyl Alcohol (John Wiley, London) 1973.
[10]. A.K. Dikshit and A.K. Nandi, Macromolecule,;31: 8886-8892, 1998.
[11]. Uma. Thanganathan, World Academy of Science, Engineering and
Technology; 71, 2010.
[12]. Y .Jin, J.C. Diniz, D.A .Costa and G.Q. Lu, Solid State Ionics, 187( 1-4):937-
942, 2007 .
[13]. S. Panero, P .Fiorenza, M.A Navarra, J. Romanowska and B. Scrosati, J.
Electrochem Soc.;152:A2400-A2405, 2005.
[14]. . M. Ahmad*Hamed, H. Sabeeh** Sabah, A Hussen*Sarkawt, Asian
Transactions on Science & Technology:1 (6):16-20, 2012.
[15]. B.S. Furnis, A.J .Hannaford, P.W.G Smith and A.R, Tatchell Vigelpartical organic chemistry ,5th ed. (ELBS, Longman Group UK,Ltd, Essex) 1991.
[16]. A.R .Patel, P.R Vavia, J. Pharm Pharmaceut Sci. ;13(2): 114-127, 2010.
[17]. S.N.Bhad, V.S.Sangawar, BionanoFrontier.; 5 (2): 186-188, 2012.
[18]. S.N .Bhad, V.S Sangawar, Vidarbha J. of Science;6(3-4): 18-23 ,2011.
[19]. P.U Asogwa, J.Optoelectronics and Biomedical Materials,2( 3): 109-117 ,2010
.
[20]. S .Erat and H .Metin, Sixth International Conference of the Balkan Physical
Union, 249 , 2007.
[21]. A .Djelloul, K. Bou, A .Zid, F. Guerrab,Turk J.Phys. 32(1) 2008.
[22]. S .Jana, R,R Thapa , R ..Maity, K.K .Chattopadhyay, Physica E. doi:101016/J.physe.2008.04.015.
IJSER © 2013 http://www.ijser.org