International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1480
ISSN 2229-5518
Efficient and environmentally friendly methods for producing hydrogen are important as the world explores the use of hydrogen as a clean energy source. The thermochemical cycles at high temperatures from nuclear power plants or concentrated solar energy are very promising methods for hydrogen production.
Water is the ideal source of hydrogen because of its abundance, low cost and the absence of CO2 emission. Thermochemical water-splitting consists of the conversion of water into hydrogen and oxygen by a series of endothermic and exothermic chemical reactions. The net reaction is equivalent to :
H2O → H2 + 1/2 O2 , with ΔH = 286 KJ/mol(1,2).
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1481
ISSN 2229-5518
The advantage of using a thermochemical reaction to produce hydrogen over the classical alkaline water electrolysis, is the low efficiency of the later which is around
20% (total of 30% for electricity * 65% for water electrolysis). Theoretically, the efficiency to produce hydrogen through thermochemical reaction, when in high temperature reactors, could be as high as 50%, because it is Carnot cycle-limited, which means that high temperature improves the conversion efficiency.
After the first cycle of water-splitting (Vanadium-Chlorine) was proposed in 1964, more than 2000 potential thermochemical cycles have been tested and checked in terms of appropriate reaction temperature, reaction velocity and economic aspects. Major problems arise from the large material flows, introduction of impurities, and by
the potential creation of toxic and environmentally unacceptable species(3-5). The final
preference of these thermochemical cycles gave about 25 cycle. Table 1 gives some of them.
The aim of this work is to study the preparation conditions of the Nickel Manganese
Ferrite for the thermochemical water-splitting and hydrogen production applications.
Cycle name | Temp., (ºC) | Reaction |
Sulfur –Iodine cycle(6), | 850 120 450 | H2SO4 → SO2 + H2O + 1/2 O2 I2 + SO2 + 2H2O → 2HI + H2SO4 2HI → I2 + H2 |
Julich Center EOS(7) | 800 700 200 | 2Fe3O4 + 6FeSO4 → 6Fe2O3 + 6SO2 + O2 (g) 3FeO + H2O → Fe3O4 + H2 (g) Fe2O3 + SO2 → FeO + FeSO4 |
AachenUniv. Julich(7),1972 | 850 170 800 | 2Cl2 (g) + 2H2O (g) → 4HCl (g) + O2 (g) 2CrCl2 + 2HCl → 2CrCl3 + H2 (g) 2CrCl3 → 2CrCl2 + Cl2 (g) |
Gas de France(7) | 725 825 125 | 2K + 2KOH → 2K2O + H2 (g) 2K2O → 2K + K2O2 2K2O2 + 2H2O → 4KOH + O2 (g) |
Tokyo Inst. Tech.Ferrite(8) | 1000 600 | 2MnFe2O4 + 3Na2CO3 + H2O → 2Na3MnFe2O6 + 3CO2(g) + H2(g) 4Na3MnFe2O6 + 6CO2 (g) → 4MnFe2O4 + 6 Na2CO3 + O2 (g) |
Nickel Ferrite(9) | 800 800 | NiMnFe4O6 + 2H2O → NiMnFe4O8 + 2H2 (g) NiMnFe4O8 → NiMnFe4O6 + O2 (g) |
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1482
ISSN 2229-5518
LASL – U(7) | 25 250 700 | 3CO2 + U3O8 + H2O → 3UO2CO3 + H2 (g) 3UO2CO3 → 3CO2 (g) + 3UO3 6 UO3 (s) → 2 U3O8 (s) + O2 (g) |
UT – 3 Univ. of Tokyo(10) | 600 600 750 300 | 2Br2 (g) + 2CaO → 2CaBr2 + O2 (g) 3FeBr2 + 4H2O → Fe3O4 + 6HBr + H2 (g) CaBr2 + H2O → CaO + 2HBr Fe3O4 + 8HBr → Br2 + 3FeBr2 + 4H2O |
US – Chlorine(7) | 850 200 500 | 2Cl2 (g) + 2H2O (g) → 4HCl (g) + O2 (g) 2CuCl + 2HCl → 2CuCl2 + H2 (g) 2CuCl2 → 2CuCl + Cl2 (g) |
Ispra Mark 9(11) | 420 150 650 | 2FeCl3 → Cl2 (g) + 2FeCl2 3Cl2 (g) + 2Fe3O4 + 12HCl → 6FeCl3 + 6H2O + O2 (g) 3FeCl2 + 4H2O → Fe3O4 + 6HCl + H2 (g) |
Ispra Mark 2(11) (1972) | 100 487 800 | Na2O.MnO2 + H2O → 2NaOH (a) + MnO2 4MnO2 (s) → 2Mn2O3 (s) +O2 (g) Mn2O3 + 4NaOH → 2Na2O.MnO2 + H2O + H2 (g) |
Ispra Mark 3(21) | 850 170 200 | 2Cl2 (g) + 2H2O (g) → 4HCl (g) + O2 (g) 2VOCl2 + 2HCl → 2VOCl3 + H2 (g) 2VOCl3 → Cl2 (g) + 2VOCl2 |
Ispra Mark 6(11) | 850 170 700 420 | 2Cl2 (g) + 2H2O (g) → 4HCl (g) + O2 (g) 2CrCl2 + 2HCl → 2CrCl3 + H2 (g) 2CrCl3 + 2FeCl2 → 2CrCl2 + 2FeCl3 2FeCl3 → Cl2 (g) + 2FeCl2 |
Ispra Mark 8(11) | 700 900 100 | 3MnCl2 + 4H2O → Mn3O4 +6HCl + H2 (g) 3MnO2 → Mn3O4 + O2 (g) 4HCl + Mn3O4 → 2 MnCl2 (a) + MnO2 + 2H2O |
Ispra Mark 1C(11) | 100 900 730 100 | 2CuBr2 + Ca(OH)2 → 2CuO + 2CaBr2 + H2O 4CuO (s) → 2Cu2O (s) + O2 (g) CaBr2 + 2H2O → Ca(OH)2 + 2HBr Cu2O + 4HBr → 2CuBr2 + H2O + H2 (g) |
Ispra Mark(11), CO/Mn3O4 | 977 700 700 | 6Mn2O3 → 4 Mn3O4 + O2 (g) C (s) + H2O (g) → CO (g) + H2 (g) CO (g) + 2 Mn3O4 → C + 3 Mn2O3 |
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1483
ISSN 2229-5518
Stoichiometric amounts of pure oxides (99.9purity of commercially available powders) of Fe2O3, NiO and MnO were mixed to prepare the spinel NiMnFe4O6 by the conventional solid state reaction technique.
The mixed oxides were calcined at 1000oC/24h in air. The calcined powders were
ground and then pressed using a uniaxial press (Hydraulic press – Perkin Elmer) to form pellets of 1cm diameter. The pressed pellets were then sintered at 1200oC/2h. The phases in the sintered material were analyzed using X-ray diffraction [(XRD) -
3A Shimadzu – Japan].
The sintered material was then ground and divided into two parts. Two different additives were used to act as supporters for the ferrite. The first was 8 mol% Y2O3 zirconia and the second was Ceria -20mol.% gadolinia, to explore the effect of supporting porous material. The mole ratios of the Zirconia support were 2 and 4 to 1 mole of the spinel ferrite, and 4 mole Ceria – gadolinia also to 1mole of the spinel ferrite. 8 wt% of starch was added as a binder for all samples. Pellets were prepared from the three mixed materials – spinel / support – powder by pressing and then
sintering at different temperatures ranging from 850 oC to 1200 oC.
Thermogravimetric measurements were performed using a TG analyzer (Shimadzu, TG – 50A). The microstructures of the different samples was examined by the Scanning Electron Microscope (SEM), [JEOL].
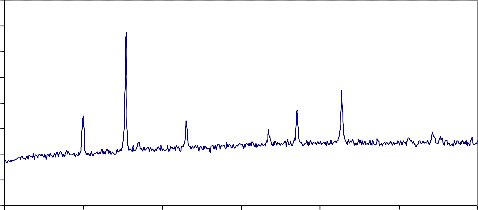
The solid state reaction between the three oxides Fe2O3, NiO and MnO took place during sintering at 1200 oC / 2h. The product had the spinel structure of NiMnFe4O8 as shown in the XRD pattern, Fig 1.
Y.Tamaura et al.(12) investigated the thermal activation of the spinel-type NiMnFe-
4O8 and the subsequent water-splitting reaction which are given by: NiMnFe4O8 →(activation at > 800°C) → NiMnFe4O8-x + x/2O2 . NiMnFe4O8-x + xH2O → (water-splitting at < 800°C) → NiMnFe4O8 + xH2
In the first endothermic step, NiMnFe4O8 is thermally activated above 800°C to form
an oxygen deficient ferrite. An important factor is the dissociation of the material to release oxygen. So that, when used in the second stage it will absorb oxygen from steam and release the hydrogen. Thermogravimetric analyses were performed on
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1484
ISSN 2229-5518
supporting materials to study their effect as well as the effect of sintering temperature on the weight loss of the material.
The thermo gravimetric analysis of Y. Tamaura and M. Tabata(13) on the ferrite
samples showed 0.3% weight loss during the activation step in the temperature range
700°C to 1100°C, with a max. rate for oxygen evolution at temperature 820°C to
827°C.
NiMnFe4O8
100
90
80
70
60
50
40
30
20

20 30 40 50 60 70 80
2^Thita
T.Kodama and N.Gokon(2), in their Nickel Manganese Ferrite system proposed a two step water-splitting reaction at 800°C, and reported that this cycle requires more moderate reaction temperature than those of the normal ferrite processes. However the amount of hydrogen evolved in this system is very limited because water-splitting was caused by the small magnitude of the nonstoichiometry in the spinel type ferrite as compared to that accompanying the Fe3O4 / FeO phase transition in the normal ferrite processes.
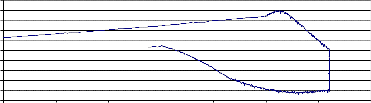
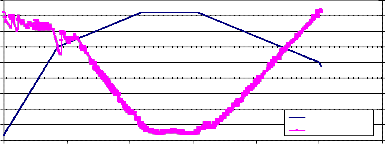
Fig. (2) shows the Thermogravimetry (T.G.) of the sample NiMnFe4O8 + 4mole 8- YSZ, sintered at 850°C, while fig. (3)shows the TG of the same sample measured at a lower heating rate. The weight loss started at about 730°C, and continued during the holding time at 820°C. During the cooling of the sample, the weight increased but did not return to its value at the beginning of heating. This may be attributed to the effect
of mixing Zirconia with NiMnFe4O8. Fig. (4)shows the weight loss of NiMnFe4O8+
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1485
ISSN 2229-5518
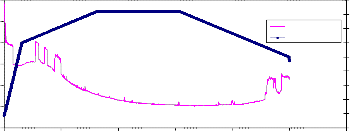
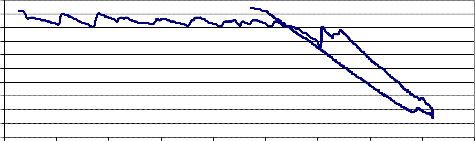
4mole Ceria gadolinia sample sintered at 850°C. The weight loss of the sample started at about 650°C, and continued during the holding time at 820°C. During the cooling the sample weight increased, but also did not return to the original weight at the beginning heating.
The samples sintered at 850°C were very brittle most probably because this temperature was not enough to complete the sintering process. However when going to higher temperatures all the sintered samples showed no weight loss. This may be attributed to closing the pores of the sintered material when it is heated at temperatures above 850°C.
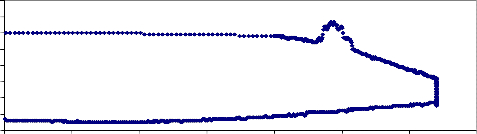
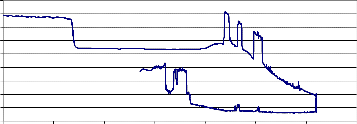
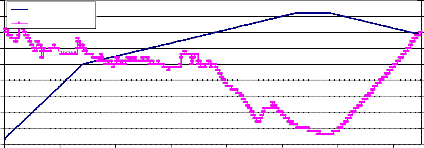
Fig. (5) shows the weight loss of NiMnFe4O8 + 2mole 8YSZ sample sintered at
900°C. The sample was well sintered, but it gave the result of fig.(5) for the first test only. Upon repeating the test for this sample, the result was zero weight loss, which may be attributed also to closing the pores of the treated material when it was heated in temperature 880°C (the holding temperature during the test).
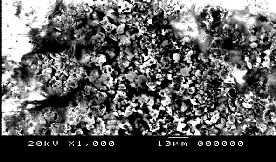
Fig. (6) represents The SEM of samples of NiMnFe4O8 + 2mole 8YSZ sintered at different temperatures 850°C, 900°C and 1200°C. It can be seen from the figures that the pores volume decreased with the sintering temperature.
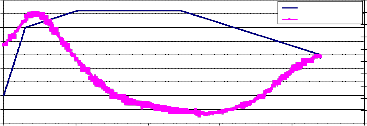
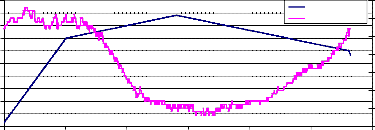
Fig. (7) represents the weight loss of pure NiMnFe4O8 powder, which started at a temperature about 660°C, and returned to its original value upon cooling at a temperature 550°C, with a maximum holding temperature at 820°C.
Fig. (8) represents the weight loss of Zirconia – 8Y powder, which started at a temperature about 700°C, and returned to its value at a temperature 700°C also, with a maximum holding temperature at 820°C.
The first step for water splitting will be heating the material, Zirconia with NiMnFe4O8 , in a vacuum at 800°C. The Zirconia deficient in the vacuum will play a good effect in taking any oxygen from the Nickel manganese ferrite. The addition of steam will produce hydrogen. If we put a Palladium screen to take the hydrogen
spontaneously, then this device can developed to work spontaneously.
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1486
ISSN 2229-5518
NiMnFe O + 4mole YSZ sintered at 850oC
900
800
700
600
500
400
300
200
100
0
Temp.,C Weight loss
226
224
222
220
218
216
214
212
210
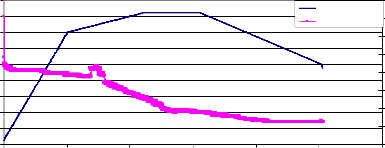
0 2000 4000 6000 8000 10000 12000
Time ,sec
Weight loss of NiMnFe O + 4 mole YSZ sintered at 850oC
220
219
218
217
216
215
214
213
212
500 550 600 650 700 750 800 850
Temp., oC
NiMnFe O + 4mole YSZ sintered at 850oC
900
800
700
600
500
400
300
200
100
0
Temp.,C Weight loss
2
1.5
1
0.5
0
-0.5
-1
-1.5
-2
-2.5
-3
0 5000 10000 15000 20000 25000
Time, sec.
NiMnFe oxide + 4mole YSZ sintered at 850 oC
2
1.5
1
0.5
0
-0.5
-1
-1.5
-2
-2.5
-3
200 300 400 500 600 700 800 900
Temp., oC
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1487
ISSN 2229-5518
NiMnFe oxide + 4CeGd oxide
0
-5
-10
-15
-20
-25
-30
Weight loss
Temp., C
900
800
700
600
500
400
300
200
100
0
Temp., oC
0 5000 10000 15000 20000 25000 30000
Time, sec.
NiMnFe O +4mole CeGd oxide sintered at 850oC
-8
-10
-12
-14
-16
-18
-20
-22
-24
-26
200 300 400 500 600 700 800 900
Temp., oC
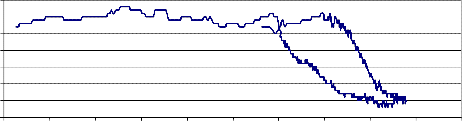
NiMnFe O + 2mole 8-YSZ, sintered at 900oC
1000
900
800
700
600
500
400
300
200
100
0
Temp., C Wight loss
318.5
318
317.5
317
316.5
316
315.5
315
0 2000 4000 6000 8000 10000 12000
Time , sec.
NiMnFe O + 2 mole 8-YSZ, sintered at 900oC
318.5
318
317.5
317
316.5
316
315.5
315
0 100 200 300 400 500 600 700 800 900 1000
Temp., oC
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1488
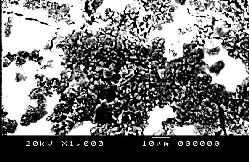
ISSN 2229-5518
NiMnFe4O8+ 2mole YSZsintered at 900°C | NiMnFe4O8+ 2moleYSZsintered at 1200°C |
NiMnFe4O8+ 2moleYSZ sintered at 850°C | NiMnFe4O8+ 4moleYSZ sintered at 850°C |
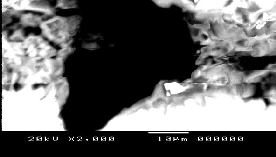
NiMnFe4O8 as a powder
900 | 216 | ||
800 | 215 | ||
700 | 214 | ||
600 | 213 | ||
500 | 212 | ||
400 | 211 | ||
300 | 210 | ||
200 | 209 | ||
Temp.,C |
100
0
Weight loss
208
207
0 2000 4000 6000 8000 10000 12000
Time, sec.
NiMnFe4O8 as a powder
216
215
214
213
212
211
210
209
208
207
206
0 100 200 300 400 500 600 700 800 900
Temp., oC
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1489
ISSN 2229-5518
T.G. of YSZ (Zirconia-8Y) powder
900
800
700
Temp., C Weight loss
192
191.5
191
600
500
400
300
200
100
0
0 1000 2000 3000 4000 5000 6000 7000
Time, sec.
190.5
190
189.5
189
188.5
188
187.5
YSZ (Zirconia- 8Y) powder
191
190.5
190
189.5
189
188.5
188
187.5
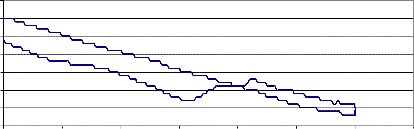
700 720 740 760 780 800 820 840
Temp., oC
The Nickel Manganese Ferrite, “NiMnFe4O8”, is a promising material for hydrogen production as a thermochemical water-splitting. The optimum working temperature of the ferrite lies between 820°C and 830°C as a maximum to avoid closing the pores or the complete sintering of the material. Mixing this material with Yettria Stabilized Zirconia (YSZ) can enhance the removal of oxygen from the Nickel Manganese Ferrite, “NiMnFe4O8”, at high temperature in vacuum.
1. B.C.R. Ewan, R.W.K. Allen; Int.J.Hydrogen Energy; 30(2005), p. 809.
2. T. Kodama, N.Gokon; “Thermchemical cycles for high temperature solar hydrogen production”; Chem. Rev.; 107(2007), p. 4048-4077.
3. H. Barnert; “Anmerkungen zur thermochemischen productions von wasserstoff aus wasser mittels hochtemperaturreaktor-wärme”, Report Jülich (1980).
4. S.H. Jensen, P.J. Larsen, M. Magensen; Int.J.Hydrogen Energy; 32(2007), p. 3253.
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1490
ISSN 2229-5518
5. A. LeDuigou, J.M. Borgard, B. Larousse, D. Doizi, R. Ewan; Int. J. Hydrogen
Energy; 32(2007), p. 1516.
6. G.E.Besenbruch, Am. Chem. Soc., Div. Pet. Chem., Prepr. 271(1982), 48.
7. L.O.Williams, Hydrogen Power, Pergamon Press Amsterdam, Netherlands (1980).
8. R.Ueda, H.Tagawa, "Production of hydrogen from water using nuclear energy",
A review, Japan Atomic Energy Res. Inst., Tokyo, Japan, 69(1974).
9. Y.Tamaura, A.Steinfeld, "Production of Solar hydrogen by a novel, 2-step, Water
– Splitting Thermochemical Cycle", Energy (Oxford) 20, 325 (1995).
10. K.Yoshida, H.Kameyama, "A simulation study of the UT-3 Thermochemical
Hydrogen Production Process", Int. J. Hydrogen Energy 15, 171 (1990).
11. G.E.Beghi, "A decade of research on thermochemical hydrogen at the joint
research center, Ispra", Int. J. Hydrogen Energy 11, 761 (1986).
12. Y. Tamaura, A. Steinfeld, P.Kuhn and K.Ehrensberger; “Production of solar
hydrogen by a novel 2 steps, water-splitting thermochemical cycle”; Energy vol.
20 no. 4 (1995), pp. 325-330.
13. Y. Tamaura, M. Tabata, Nature, vol. 346 (1990), p. 255.
IJSER © 2014
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 1491
ISSN 2229-5518
: ة مد قملا
ةيرارحلا ءايميكلا ةطساوب نيجورديهلا جاتنأو ءاملا لصفل ىديدحلا زيناجنم لكينلا
لف دلقو , .م° 088 ةرارل ةلجرد طل نيفولطت طل ءالملا للصفل ماظنك ىديدحلا زيناجنم لكينلا ةدام تسرد ىروللبلا اليكرتلا ولكتيل .م°0088 ةرارل ةلجرد ط ةئ اكتملا ةيئاميكلا اهبسنب ةبلصلا اهتلا ط ديساكلأا لعافف
ل تلتبه دلقو ,ةليدويحلا ةينيلسلا ة لعلأا مادختلسأب ا دل ر لف طلتلاو ىدليدحلا زينالجنم للكينلاب صاخلا لنيبسلأا الينوكرزلاو اليرتيا الينوكرزلا نلم نيعولن نلم هنم ةيماسم تانيع عينصف ف و كيماريس اينوكرزلا ةدام عم داوملا ةيمالللسملا داولللملا ة لللهل نيخلللستلاب ةدولللقفملا اوولأا ةلللسارد لللف دلللقو .ةيمالللسملا الللينوكرزلا ريهألللف ةلللبرجتل ايريلللس
.اهقي اسمو
IJSER © 2014