International Journal of Scientific & Engineering Research, Volume 5, Issue 6, June-2014 755
ISSN 2229-5518
Jojoba and Castor Oils as Fluids for the prepara- tion of bio greases: A Comparative Study
El-Adly R. A., Ahmed H. Bedier, Modather F. Hussein, Enas A. Ismail and Mahmmoud M. El-Emary.
Abstract— The search for environmentally friendly materials that have the potential to replace mineral oil in various industrial applications is considered a priority research in the fields of lubricants, fuels and energy sectors. Accordingly, vegetable oils seem to be significant potential base fluids and as a substitute for mineral oils as greases formulations. The aim of this study is to investigate the comparison of jojoba and castor oils as base fluids for producing bio- based greases. In this respect, the sulfurization of jojoba and castor oils was carried out at temperatures ranging from 110 to 170oC. The physicochemical properties of the sulfurized jojoba and castor oils were found be better than the non-sulfurized oils. The flow and the viscoelastic properties of these oils had been studied by the programmable Rheometer
HADV-III ultra- system that was operated in either a steady rotation or in an oscillatory mode. The obtained data revealed that the flow behavior depended to a large extent on the nature of the aforementioned oils. Jojoba oils exhibited properties close to castor oil except for the oxidation stability which was better for jojoba oil. The influence of these oils and their sulfurized products on the properties of the prepared bio greases was studied. The data obtained in this investigation indicated that the prepared bio greases from the sulfurized oils had superior properties with respect to the dropping point and the consistency as compared to the non- Sulfurized oils. The prepared greases from the jojoba oil and its sulfurized product showed an improvement in the oxidation stability than the corresponding greases based on the castor oil or its sulfurized product. An opposite trend was observed concerning the consistency and the flow properties. It was concluded that the jojoba and castor oils showed appropriate properties to be used as fluids for bio lubricating greases. In addition, the performance characteristics of the obtained greases were largely dependent on the type of the base fluid.
Index Terms— Jojoba oil, Castor oil, Sulfurization , Rheology, Bio lubricant and Bio greases.
—————————— ——————————
1 INTRODUCTION
ithin the area of alternate sources of lubricants, a new frontier remains for researchers in the field of lubricating greases. Lithium greases have good multi-purpose prop- erties, e.g. high dropping point, good water resistance
and good shear stability. The preparation, evaluation and de-
velopment of lithium lubricating greases from low cost start-
ing materials such as, bone fat, cottonseed soapstock and jojo-
ba meal was explored (1-5).
The role of the jojoba oil and its meal as novel additives for sodium lubricating greases was investigated (6). Jojoba oil may be considered a low-energy replacement for conventional fats and oils The chemical composition of jojoba oil is unique in that it contains little or no glycerin and that most of its com- ponents fall in the chain-length range of C36 -C42 . Linearity and close-range composition are probably the two outstanding properties that give jojoba oil its unique characteristics. Jojoba oil molecules contain two double bonds separated by an ester bond. These three active centers have been proven to be the source of a very large number of intermediates or final prod- ucts (7).
ـــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــ
• Author name is
Refaat A. El-adly, professor of petroleum chemistry in Egyptian petroleum
Research Institute, Cairo, Egypt., PH 00201224892927. E-mail: au- thor_refeladly@hotmail.com
• Co-Author name is
1-Ahmed Hhammam Bedair, professor of Organic chemistry in Faculty of
science, Chemistry Department, Al-Azhar University, Cairo, Egypt.
2- Modather F. Hussein, Lecture in Faculty of science, Chemistry Depart-
ment, Al-Azhar University, Assuit branc.
3- Enas A. Ismaelm, Researcher in Egyptian petroleum Research Institute,
Cairo, Egypt.
4- Mahmmoud M. El-Emary, professor of Applied chemistry in Egyptian
petroleum Research Institute, Cairo, Egypt.
The castor oil plant (Ricinus communis), a member of the large spurge family (Euphorbiaceae), is a native of tropical Asia and Africa. Castor oil has long been known as medicinal oil and was primarily used as purgative or laxative to counter consti- pation (8) .
Moreover, it possesses nauseate properties and is classified as non-edible oil. The plant was already grown for its oil in
Egypt some 6000 years ago. Nowadays, it is naturalized and cultivated on commercial scale all around the world in tem- perate zones. Asia can be considered as the main player for oils and fats used for the oleochemical industry (9-11). Lubricating greases are widely used in mechanical compo- nents of various kinds of machines. Greases are solid or semi- fluid lubricant. They basically consist of liquid lubricant and thickening agent which constructs fibrous microstructures in lubricant. Other ingredients which produce special properties may be included (12-14). Because of this two-phase system, the special features of greases such as the long interval of lubri- cant supply or the simple sealing mechanism are induced. Environmentally friendly lubricants and greases are already on the market (15). These products are very desirable in total loss lubricants such as railroads. Certain synthetic greases, which are based on low molecular weight poly ά –olefin, pol- yglycols, polyol esters, and diesters, are biodegradable and environmentally friendly, but their higher cost limits their applications only in niche areas such as aerospace, computer, and medical applications (16).
In this respect, vegetable oil based greases have poor ther-
mo-oxidative stability and thus cannot be used at high tem- peratures. Dwivedi et al. (17) described the preparation of total vegetable oil based grease using castor oil. Florea et al. (18) have
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 6, June-2014 756
ISSN 2229-5518
studied the effect of different base fluids on the properties of biodegradable greases. In most applications, a suitable compo- sition of grease is desired with good performance properties capable of use in multifunctional products. Despite the over- whelming importance of biodegradable greases, very little is known about the relationship between their composition and performance properties. Soybean oil has also been used by American researchers for manufacturing soy grease for lubri- cation heavy duty truck. Unfortunately, research and or man- ufacturing of such a biogrease from jojoba and castor oils are rarely reported. This paper is an attempt to understand how the composition of jojoba, castor oils and their sulfurization in biobased grease affects on their properties.
2. PROCEDURE FOR PAPER SUBMISSION
2.1 Materials and techniques
Jojoba and castor oils were obtained locally as the biodegradable fluids for preparing bio greases under investigation. Jojoba oil was supplied by the Egyptian National Oil Company and castor oil was supplied by El-Nasr pharmaceutical chemical Co. Jojoba and castor oils are designated as JO and CO, respectively. Lithi- um stearate was kindly supplied by Morgan Co.
Oxidation assessment for the jojoba and the castor oils were
determined according to the method describe by Tod et al (19). In
this respect, a sample of oil was heated at 100°C while air was
bubbled through it and the volatiles created were transferred to
a water trap where the conductivity was measured .The induc- tion period endpoint was determined by the time it took for the sample to begin a rapid increase in the conductivity. The time required for the sample to reach its induction period endpoint was termed the Oil Stability Index (OSI). The peroxide values and iodine values were determined using the International standard method.
The average molecular weight was measured by gel permea-
tion chromatography (Water 600E) equipped with styagel col-
umn operated at 40oC and a flow rate of 0.4 ml/min. The re-
fractive index instrument model Water 4110 and toluene
(HPLC grade) were used as a mobile phase.
The thermo gravimetric analysis for oils under investigation
was carried out in a TA Instruments SDTQ 600 simultaneous
TGA-DSC thermo gravimetric analyzer. The analyzers were
conducted for a total sample mass of 16.0 ± 0.4 mg. A known
amount of the sample was loaded and evenly spread on the alumina micro crucible. The samples were heated under nitro- gen flow (100 ml min-1) from 50 to 550°C, at 5 °C/ min. Rheological characterization for the samples under investiga- tions was performed on a Brookfield programmable Rheome- ter LV DV-III UITRA used in conjunction with Brookfield software, RHEOCALC V.2. Through RHEOCALC, all Rheom- eter functions (rotational speed, instrument % torque scale, time interval, set temperature) were controlled by a computer. The corresponding shear stress, shear rate, dynamic viscosity and consistency index were also recorded through the soft- ware. The temperaturewas controlled by connection with a bath controller HT-107 and measured by the attached temper- ature probe. Before carrying out the measurements, the Rhe- ometer DV-III UITRA should be turned on, leveled and auto zeroed. The level was adjusted using the three feet on the bot-
tom of the base and was confirmed using the bubble on the top of the head. The feet was adjusted until the bubble was inside the center target and the level was set prior to auto zero. Eight grams of the tested sample were placed in the cup (chamber) of the apparatus. The spindle used was SC4-18 con- trollinthe cooling and heating through the refrigerat- ing/heating circulating bath.
The rheological behavior and the flow properties of the jojoba
and castor oils were carried out at different temperatures namely, 40oC, 60oC, 80oC and 100oC; while for the prepared greases were carried out at 110oC.
Fatty acid analysis for JO and CO were identified using Ag- ilent 6890 series GC apparatus provided with a DB-23 column (60m×0.25µm). Fatty acids were transformed into their methyl
esters and were directly injected in to the GC. The carrier gas was NR2R with a flow rate of 2.2 ml/min. The injector tempera- ture was 250oC and that of FID detector was 270oC .The pro- gram temperatures setting were as follows: 150oC to 225oC at
50oC/min, and then held at 225oC for 20min.
2.2. SYNTHESIS OF THE SULFURIZED JO & CO
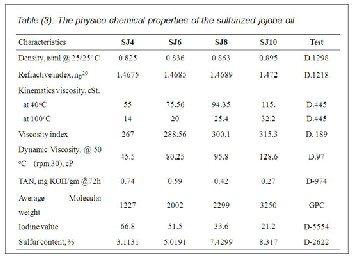
Jojoba molecules contain two double bonds, also, castor oil contain three double bonds in its glycerides molecules. These bonds are considered active sites for many reactions. Accord- ingly, sulfurization of jojoba and castor oil were carried out by the addition of 4%, 6%, 8% and 10% weight of elemental sul- fur. The obtained sulfurized jojoba are designated as SJ4, SJ6, SJ8 and SJ10 respectively while the sulfurized castor oil are designated as SC4, SC6, SC8 and SC10 respectively.
The total sulfur for each test, to be added, was divided into four portions. Each portion was added at temperatures 110,
130, 150, and 170oC followed by stirring while nitrogen gas was flushed. To ensure that all the added sulfur was reacted, the products obtained were tested using a filter paper wetted with lead acetate. The physicochemical properties of the ob- tained sulfurized jojoba and castor oils were carried out ac- cording to standard methods (21-22).
The sulfur content in each sample was determined by X-ray
fluorescence model spectro phoenix II.
2.3. GREASES PREPARATION
Four grades from bio based greases were prepared based on Jojoba oil, Castor oil, Sulfurized jojoba (SJ8) and Sulfurized castor (SC8) designated as G1, G2, G3 and G4, respectively. Mean while, thickening agent for these greases was lithium stearate. These biogreases were prepared according to a pro- cedure described elsewhere (23). The physicochemical proper- ties of the obtained bio greases were determined according to ASTM methods as mentioned in table 4.
3. RESULTS AND DISCUSSION
There had been a lot of interest in using vegetable oils as renewable raw materials for new industrial products including lubricants. This emphasis on environmentally friendly lubri-
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 6, June-2014 757
ISSN 2229-5518
cants was largely due to the rapid depletion of world fossil
fuel reserves and increasing concern for environmental pollu-
tion from the excessive mineral oil use. Jojoba and castor oils
are promising candidates in this study as base fluids for pre- pared bio based grease. Jojoba is unique among plants in that its nuts contain about 50% by weight of a practically odorless, colorless oil compound mainly of the straight chain monoes- ters of C20 and C22 alcohols and acids, with two double bonds, one at each side of the ester bond. The almost complete ab- sence of glycerin indicated that jojoba differed radically from all other known seed oils: it is not a fat but a liquid wax (24).
In this respect, the physicochemical properties of local jojoba and castor oils were determined using ASTM/IP standard test methods and are summarized in Table 1. Generally, the perox-
ide value and total acid number are used as an index of the degree of oxidative rancidity of the vegetable oils. The exper- imental data in Table 1, showed that the jojoba oil had lower values for both peroxide value and total acid number as com- pared with castor oil. In addition, the oxidation stability in- dexes for the JO and CO were 51 and 19, respectively. This revealed that the degree of oxidative rancidity of jojoba oil was lower than in castor oil. This was due to the role of the chemical structures of jojoba oil and its natural antioxidants such tocopherol (24-25). On other hand, the density of jojoba oil was low as compared with castor oil because jojoba oil is actu- ally a wax comprised of fatty alcohols and acids. Based on this findings jojoba oil had a high viscosity index as compared with castor oil.
Inspection of the data of the physicochemical properties shown in Table (1), revealed the possibilities for the develop-
ment of these oils under investigation and their suitability to produce lubricants to be used in the preparation of lubricating greases.
The chemical compositions of the jojoba and castor oils were investigated using GC model. The analyses were used to de-
termine the fatty acids. The obtained data in Table 2, showed that the identified fatty acids in jojoba oil were lauric acid, palmetic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, eicosenic acid, deocosenoic acid, Tetra- cosenoic acid and Hexacosenoic acid with percentages;
0.03,1.62, 0.6, 0.17, 10.11, 0.19, 0.25, 57.13 , 11.36, 10,6 and 8.46 respectively. This indicated that the main components were eicosenic and deocosenoic acids. The results obtained agreed with those reported by Miwa (26)
This proved that the chemical composition of jojoba oil was
unique in as much as it contained little or no glycerin and that most of its components fell in the chain-length range of CR36-42R. Linearity and close range composition are probably the two out- standing properties that gave jojoba oil its unique characteristics. In the meantime, jojoba oil molecules contain two double bonds separated by an ester bond. These three active centers had been proven to be the source of a very large number of intermediates or final products.
In addition, Table 2 showed that the identified fatty acids in castor oil are ricinoleic acid, palmetic acid, stearic acid, oleic acid, linoleic, linolenic acid and eicosenic acid with percent- age; 83.6, 1.1, 1.92, 4.11, 6.76, 0.65 and 0.5 respectively. In gen- eral, the obtained data showed that the fatty acids composi- tions in the jojoba and castor oils depended on the origin of the planting oil, climatic conditions and soil composition.
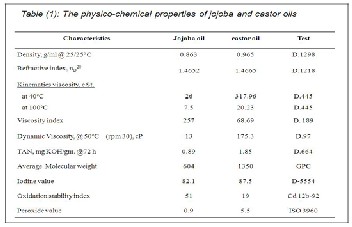
The rheological behavior of jojoba and castor oils had been
determined by the viscometric shear rate and the shear stress
rate measurements at 40o, 60o, 80o and 100oC, are shown in

Figures 1& 2. These figures showed that the flow behavior for the above mentioned oils were Newtonian. With respect to the variation in the shear stress as corresponded to the shear rate; castor oils showed higher variations than the jojoba oil, indicating that the force applied to shear and yield stress of the castor are higher. This view is in agreement with the dy- namic viscosity data for local jojoba and castor oils as shown in Tables 1. It may be explained that the shear applied in cas- tor oil broke down the internal structure within the bulk (tri- glycerides) rapidly and was temperature dependent. Also, the increase in temperature tended to increase the molecular motion and reduce the attractive forces between the mole- cules.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 6, June-2014 758
ISSN 2229-5518
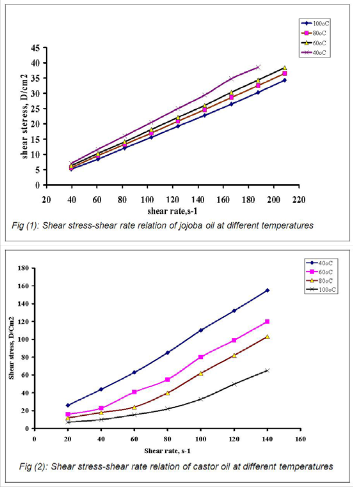
Accordingly, jojoba was close to Newtonian behavior and was weekly dependent on temperature as compared with castor oil.
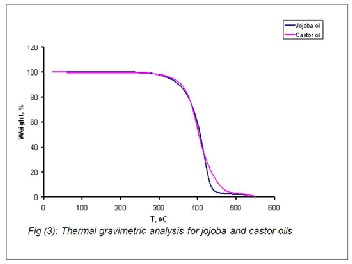
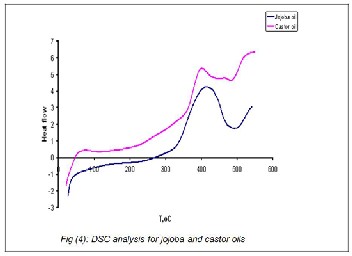
The thermal stability and phase transition of the vegetable oils had attracted the attention of several researchers (27-28). It was therefore of interest to investigate the thermal behavior of the jojoba oil as compared with to castor oil. The TG\DSC thermo grams of jojoba and castor oils are shown in Figures 3
& 4. Careful inspection of these Figures showed that the weight changes and thermal activities in terms of the exo- thermic or the endothermic heat flows during the oxidation reactions. The TG thermo grams (Figure 3) did not clearly dif- ferentiate between the jojoba and the castor oils. But, the DSC thermo grams (Figure 4) provided better information on the oxidation performance and the thermal transitions occurring within a temperature test ranging from zero to 500o C of both oils under study. The behavior shown in these Figures indi- cated that the isothermal TGA\DSC could be effectively used to compare the oxidative and thermal performances of such vegetable oils. In addition, the local jojoba oil showed com- paratively a better oxidative and thermal performance than the local castor oil. This revealed that the role of the degree of unsaturation double bonds and structural configuration of both oils. This view agreed with the data presented in Table
2; jojoba oil contains low percentage of the polyunsaturated
fatty acids as compared with castor oil.
The mechanism of the autoxidation of vegetable oils was well
studied(29). Vegetable oil oxidation was initiated by the for- mation of free radicals. Free radicals could easily be formed from the removal of a hydrogen atom from the methylene group next to a double bond. Free radicals rapidly reacted with oxygen to form a peroxy radical. The peroxy radical could then attack another lipid molecule to remove a hydro- gen atom to form a hydroperoxide and another free radical, propagating the oxidation process. Two compounds are ob- tained from this process.
They are containing primary oxidation compounds such as hydro peroxides and secondary oxidation compounds such as volatile organic compounds, which are formed following the decomposition of the triglyceride hydro peroxide. For this reason, the methods used to assess the oxidation stability of the vegetable oil formulations must be carefully matched to the intended application in order to obtain realistic estimates. In this respect, the changes in the chemical composition of jojoba and castor oils caused by oxidation were analyzed ac- cording to the method reported by Tod et al (19).
This method monitored the compounds resulting from the
decomposition of the highest molecular weight of the hy-
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 6, June-2014 759
ISSN 2229-5518
droperoxides, short chain fatty acids and alcohols (primary oxidation products and secondary oxidation compounds).
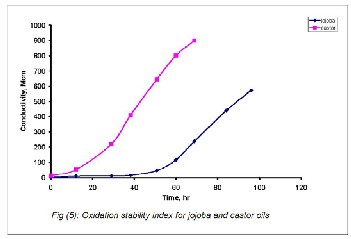
The effects of the variation in the oxidation time on conduc- tivity had been investigated from zero time to 100 hours in case of the local jojoba oil and from zero time to 75 hours for the local castor oil. The analytical data obtained are presented in Figures 5. This figure presented an overview on the quanti- ty of the volatiles compounds which resulted from the oxida- tion reaction that happened during the tested time. In case of the jojoba oil the end point observed was 51 hours. However, in the case of castor oil the end point was 19 hours.
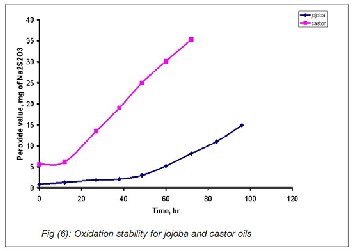
In this respect, Figure 6 represents the effect of oxidation time on the peroxide value of the jojoba and castor oils. The results showed that the peroxide values for castor oil were increased markedly after 10 hours. In contrast, in case of the jojoba oil the peroxide values were almost stable until 35 hours and slightly increased after 50 hours.
Table1, clearly indicated that the jojoba oil was, in general, more effective in controlling the oxidative deterioration and more efficient in preventing the formation of primary oxida- tion products and secondary oxidation compounds. This was attributed to the tocopherol isomers in jojoba oil which acted as an antioxidant (25,29).
Comparative investigation between jojoba and castor oils through the sulfurization reaction were carried out by the addition of different concentrations of elemental sulfur, 4%,
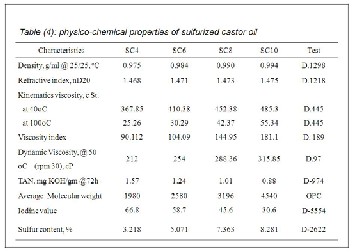
6%, 8% and 10%; the obtained sulfurized jojoba are designat- ed SJ4, SJ6, SJ8 and SJ10 respectively (Table 3), but the ob- tained sulfurized castor oils are designated SC4, SC6, SC8 and SC10, respectively (Tables 4).
The tabulated data indicated that the viscosity index, dy- namic viscosity and molecular weight increased with in- creasing the sulfur content. On the other hand, the iodine number was decreased by the increase of the sulfur content. This indicated that the sulfurization reaction temperature followed in the experimental section was suitable for such oils.
Careful inspection in the above mentioned Tables revealed
that the molecular weights concerning the sulfurized castor
oil had higher values than the corresponding sulfurized jo-
joba oil in all concentrations. This was attributed to the
chemical configuration of the castor oil, three dimensional structure of the triglyceride (32), and its double bonds which tended to increase the degree of cross linking by sulfur atoms.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 6, June-2014 760
ISSN 2229-5518
Grease evaluation
The preparation of lubricating greases was a complicated trial and error process in which the optimization of the ingredients and the reaction schemes were to achieve the desired grease consistency. Thus, the development of lubricating greases with the right consistency required stringent optimization of the components and the preparation scheme. Important perfor- mance properties such as rheology, oxidation, oil separations and dropping point were largely dependent on the grease hardness and its ability to maintain a stable lubricating film at the metal contact zone. Accordingly, four bio based greases were prepared based on jojoba oil, castor oil, sulfurized jojoba and sulfurized castor oils with 8% sulfur by weight designated as G1, G2, G3 and G4 respectively (Table 5).
It is worth mentioning that the specifications of all the pre- pared bio greases met with the National Lubricating Grease Institute-2 (NLGI-2). In the meantime, G3 and G4 had thicken- ing power over G1 and G2. This indicated that the role of the sulfurization oils used in the preparation of bio greases where the increase of molecular and dynamic viscosity that led to thickening power as compared with nonsulfurized oils.
In general, data in Table 5 show that the bio greases prepa-
ration was complicated trial and error processes in which the
optimization of the fluids, jojoba, castor and their sulfurized oils, and the chemical compositions of these oils were critical to achieve the desired grease property.The type of these oils and its composition played important roles in the physico- chemical characterization of the prepared greases.
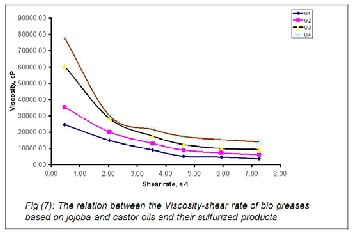
The rheological behavior of the prepared greases (G1-G4) was explored. The apparent viscosity- shear rate profiles at temperature 110oC for these greases were investigated and presented in Figure 7. From these data, it was clear that the apparent viscosity decreased approximately linearly in the order of G4 > G3 > G2> G1 in the first region up to shear rate
2.0 S-1 . After that, in the second region, the viscosity almost
remained constant with increasing the shear rate for all the
greases with the same trend. It is apparent that the rheological
properties for the prepared greases G4 and G3 were improved with the sulfurized oils. Such improvements might be attributed to the high degree of cross linked of the sulfurized castor oil than sulfurized jojoba oil. This observation was further supported by the high molecular weights of the sulfurized castor oil and sulfurized jojoba oil (Table 3 & 4), which led to the increase of both the compatibility and the electrostatic forces between the ingredients of the prepared bio greases.
.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 6, June-2014 761
ISSN 2229-5518
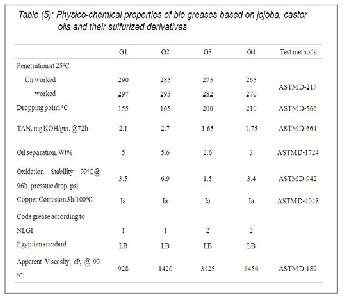
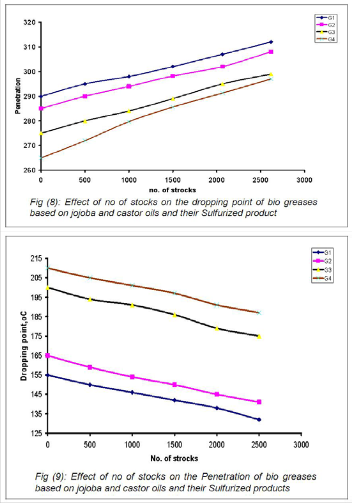
The experimental data graphically presented in Figures 8 and
9. These data showed that the variation of the dropping points
and the penetration values with different mechanical strokes
from 60 to 2500. It was obvious that the mechanical and ther- mal stabilities for the prepared greases are in the order of G4 > G3 > G2> G1. It was apparent from these Figures that the greases G2 and G4 based on castor and its sulfurized products were more efficient concerning the thermal and the mechani- cal properties than the corresponding greases G1 and G3 based on jojoba and its sulfurized product. This view agreed with the molecular weight data for CO, SC8, JO and SJ8 (Table
3 & 4) and revealed that the chemical composition role for the jojoba oils and the castor oils.
4. CONCLUSIONS
The results presented in this investigation indicated the fol- lowing conclusions:
1- The fatty acids compositions of the jojoba oils are different from those of castor oils.
2- The sulfurization reactions for the jojoba oils and the castor
oils improved their properties as greases.
3- Bio-grease based on castor oil and its Sulfurized products
showed better performances concerning the mechanical stabil-
ity as compared with the jojoba oil. However, the oxidation stability showed a reverse trend.
4- Both jojoba and castor oils were good raw materials for the synthesis of bio greases.
REFERENCES
[1]- El-Adly R. A. (1999). Producing Multigrade Lubricating Greases from Animal and Vegetable Fat By-products. J. Syn- thetic Lubrication. Vol.16, No.4, pp. 323-332.
[2]- El-Adly R. A.; El-Sayed S. M. & Ismail M. M. (2005). Stud-
ies on TheSynthesis and Utilization of Some Schiff’s Bases: 1.
Schiff’s Bases as Antioxidants for Lubricating Greases. J. Syn-
thetic Lubrication Vol.22, pp. 211-223.
[3]- El-Adly, R.A & Enas A. Ismail. (2009). Study on Rheologi-
cal Behavior of Lithium Lubricating Grease Based on Jojoba
Derivatives. 11thLubricating Grease Conference, Mussoorie,
India. February 19-21 2009 ( NLGI India Chapter)
[4]- El-Adly,R.A. (2004). A Comparative Study on the Prepara- tion of Some Lithium Greases from Virgin and Recycled Oils, Egypt J. Petrol Vol.13, No, 1. pp. 95-103.
[5]- El-Adly,R. A and Ismail, E.A,. Lubricating greases based on fatty by-products and jojoba constituents, Chapter 8, Tri- bology-lubricants and lubrication, ISBN 978-953-307-371-2,
INTECH publisher, 2011
[6]- El-Adly, R.A.; El-Sayed, S.M. & Moustafa, Y.M. (2004). A
Novel Application of Jojoba Meal as Additives for Sodium Lu-
bricating Grease, The 7th International Conference on Petrole-
um & the Environment, Egyptian petroleum Research Institute
In Cooperation with EURO-Arab Cooperation Center & Inter- national Scientists Association, Cairo, Egypt. March 27-29 2004. [7] Jaime Wisniak, Potential uses of jojoba oil and meal - a review Industrial Crops and Products 3 (1994) 43-68
[8] A. Scarpa, A. Guerci: Various uses of the castor oil plant
(Ricinus communis L.) – a review. J Ethnopharmacol. 1982,
5, 117–137.
[9] M. F. Ali: Edible oils, fats and waxes. In: Handbook of
Industrial Chemistry: Organic Chemicals.
[10] O. D. Onukwlo, P. K. Igbokwe: Production and charac-
terization of castor oil-modified alkyd resins. J Eng Appl Sci.
2008, 3, 161–165.
[11] Hatice Mutlu1 and Michael A. R. Meier: Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30
[12] Braithwaite, E.R.: Lubrication and Lubricants, Elsevier
Publishing Company (1967) 197-240
[13] Cameron, A.: Principles of Lubrication, Longmans
Green (1966) 528-541
[14] Hoshino, M.: Theory of Grease Lubrication, Journal of
Japanese Society of Tribologist, Vol.47, No.1 (2002) 8-14
[15] Sullivan, T. Soy Grease on Track for Sales Boom. Lube
Report 2003, July 22.
[16] Bessette, P. A.; Stone, D. S. Synthetic Grease (Chapter
23). In Synthetic Lubricants and High Performance Func-
tional Fluids.
[17] Dwivedi, M. C.; Sapre, S. Total Vegetable-Oil Based
Greases Prepared from Castor Oil. J. Synth. Lubr. 2002, 19
(3), 229.
[19] Florea, O.; Luca, M.; Constantinescu, A.; Florescu, D.
The Iinfluence of Lubricating Fluid Type on the Proper-
ties of Biodegradable Greases. J. Synth. Lubr. 2003, 19 (4),
303.
[20] “Collaborative Study of the Oil Stability Index Analy-
sis”. Tod A. Jebe, Mark G. Matlock and Ronald T. Sleeter. JAOCS, vol. 70, #11, pp1055-1061, 1993
[21] Official methods of analysis of the association of Agri- cultural chemists. 15th ed., published by A.O.A.C, (2000)
[22] Peeler, R. L.. Hartmann, L. M.. "Evaluation of Sulfu-
rized Sperm Oil Replacements", presented at the 40th Annu- al Meeting of the National Lubricating Grease Institute, Denver, Colo.. Oct 1972.
[23] Refaat A. El-Adly, A. H. Bedier, Enas A. Ishmael and
Modather F. H, A Study on preparation and evaluation of
biogrease based on jojoba oil and its derivatives. The 14th. International Conference On Petroleum Mineral Resources and Development ,EPRI,Nasr city, Cairo, Egypt. March, 2011 [24] Wisniak, J. (1994). Potential uses of jojoba oil and meal- a review. Industrial Crops and products.3.43-68.
[25] Kono, Y.; Tomita, K.; Katsura, H. & Ohta, S. (1981). An- tioxidant in Jojoba Crude Oil, In: Puebla, (Editor), Proceed- ings of the Fourth International Conference on Jojoba, Her- mosillo, pp 239-256.
[26] Miwa, T.K. (1971). Jojoba Oil wax Esters and Derived
Fatty Acids and Alcohols, Gas Chromatographic Analysis ,
J.Am.Oil Chem., Vol.48, pp 299-264.
[27] Jayadas N. H.(2008) Evaluation of the oxidative prop-
erties of vegetable oils as base stocks for industrial lubricants
using spectroscopic and thermogravimetric analyses, J. Syn-
thetic Lubrication 2008; 25: 105–113
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 6, June-2014
ISSN 2229-5518
[28] Dweck J, Sampaio CMS. Analysis of the thermal decom position of commercial vegetable oils in air by simultaneous TG/DTA. Journal of Thermal Analysis and Calorimetry 2004;
75:385-391
[29] Stachowiak, N.J. Fox, G.W. Vegetable oil-based lubri cants-A review of oxidation, Tribology International 40 (2007) 1035-1046
762
IJSER lb)2014
http://www.ijserorq











