spectrum and was good agreement with the most possible
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 658
ISSN 2229-5518
T. Lawrence, S. Gunasekaran
ABSTRACT: The alkaloid berberine is isolated from the leaf of Mahonia leschenaultii with various organic polar solvents and subsequent IR, UV, and MS spectral analysis on the berberine was carried out and characterized. The theoretical values of IR, UV spectral data and possible structure of the compound are obtained by the Gaussian and ChemDoodle software. These theoretical values are good agreement with the experimental values and the structure of the alkaloid confirmed.
Keywords: Isolation, Alkaloid, Mahonia leschenaultii, IR, UV, MS values.
performing Mayer’s and Dragen dorff’s reagent alkaloid test. The leaf powder dried completely from benzene and is soaked
The methanol extract of the root and root bark of mahonia
leschenaultti has rich in alkaloids [1]. The alkaloids ware isolated including emetine, veratrine, strychnine, piperine, caffeine, quinine, berberine, coniine, atropine, etc., [2]. One of principal alkaloids in this plant is berberine (1.02%) [3,4]. Although a number of synthetic routes to the berberine
in methanol for 96 hours. The methanol extract collected in air tight container. The menthol extract prepared for 2 times from the coarse powder. The collective methanol extract filtered multiple times by #1 waterman paper for removing of powder
IJSER
alkaloid have been thoroughly investigated, all of those using
readily available starting materials fail to give rise to the naturally occurring compounds without multistep reaction sequence [5]. The Todas, the Nilgiris tribe, Tamilnadu, India, are using the extract for postnatal treatment in women [6, 7]. Methanol extract of this plant is act as an anti fungal and anti bacterial medicine [8]. The berberine was isolated successively with various organic solvents [9, 10]. Biotechnological approaches have to be sought for the large scale production of the alkaloids such as berberine [11].
Mahonia leschenaultii species of Berberidaceae family is a
shrub with rough, grayish – brown, corky bark and leaves in circle present at the end of slender branches. Flowers are yellow, in long erect racemes [12, 13]. The plant leaves was collected from Parson’s Valley forest area, Udagamandalam, The Nilgiris district, Tamilnadu, India in the month of December.
3 kg of Mahonia leschenaultii leaves were collected from
healthy plant and cleaned, washed with water, removes the needles, dried for 3 days and mechanically powdered for obtained course powder. It is subjected to extraction in a Soxhlet condenser apparatus by methanol. For removing moistures such as gum, wax etc., 1.5 kg of the leaf powder is soaked in 2 liter petroleum ether for 72 hours by 3 times and the leaf separated and dried. Similarly by benzene the
moistures were removed [14]. The petroleum ether and benzene extracts does not show the alkaloid presents while
and sediments. A Soxhlet condenser arrangement is used for
evaporating the excess methanol from methanol extract by mantel arrangement at 60 0 C. The methanol evaporates up to its 1/20th volume (50 ml) from methanol extract and it is called methanol concentrate.
The methanol concentrate may contain tar, moisture, flavonoids, steroids etc. Hence, it is washed by petroleum ether and with few drops of dilute H2 SO 4 . Oil like residue removed and the methanol concentrate filter by multilayered No.1 waterman paper. Petroleum ether and methanol were evaporates and the brownish past like filtrate obtained and it was again dissolves in to the pure ethanol Then it is called methanol filtrate.
The methanol filtrate contains an alkaloid [15] was separated
by column chromatography on basic silica mesh 400, in petroleum ether. It is quick physical method for separation of individuals from mixing of compounds. This is a separation which works well for tropane alkaloids such as atropine, cocaine, and scopolamine berberine [16]. The methanol filtrate was chromatographed (SiO2, CHCl3: MeOH = 10:1) for isolate pure alkaloids. The column was packed by using the suspension of silica gel in petroleum ether. All the fractions of elutes were collected and kept in separate containers. Petroleum ether and methanol were evaporated out and all samples dissolve in pure nHexane and were named as S3/C1, S3/C2, S3/C3, S3/C5 and S4/C5.
Mayer’s reagent [17] and Dragen dorff’s reagent [18] are used
for testing the sample fractions S3/C1, S3/C2, S3/C3, S3/C4 and S4/C5. Few drops of Mayer’s regent added with 3 ml for
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 659
ISSN 2229-5518
all the samples. S3/C2 gives while precipitate and it shows that the presence of alkaloid and again S3/C2 confirms the alkaloid by Dragen dorff’s regent, obtaining brown precipitate.
The purity of S3/C2 was checked by Thin Layer
Chromatography Merck silica gel coated glass slab kept
slanting in 20ml nHexane containing 100ml closed beaker and a small dot of S3/C2 placed at mid of the plate. The paper chromatography as #1 waterman paper hanged in 20ml nHexane containing 100ml closed beaker and a small dot of S3/C2 placed at mid of the paper. TLC & PLC shows the movements of single dot. Hence S3/C2 was a single compound.
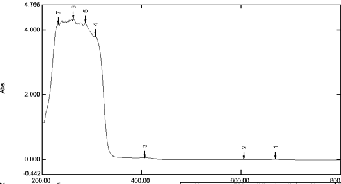
UV spectra were recorded on a SHIMADZU spectrometer at. IR spectra of samples liquid base were also recorded the
SHIMADZU Fourier transform infrared spectrometer using NaCl cells. MS spectral data were recorded by JEOL GC Mate instrument at IITM, Chennai.
The Gaussian software (V3) was used for obtaining IR, UV
spectrum and was good agreement with the most possible
molecular structure of the given IUPAC named compound of molecular diagram. The IUPAC name of berberine was obtained by ChemDoodle software .The IUPAC name of the berberine is 18,19-Dimethoxy-6.8-diolxa-i- azapentacyclohenicosa -1,3,5,10,13,15,17,19- octaene and the molecular formula is C20 H18 NO4 .Theoretical values are compared with experimental result and are listed bellow.
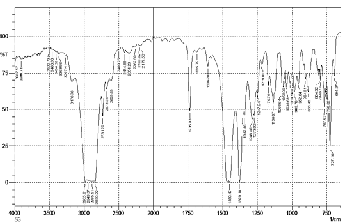
1379, C-O stretch (esters) and C-N stretch (aromatic amines)
1288, 1273, 1247, O-H bend (carboxylic acids) 983,952, 904, C-H “OOP” 883,794,767,756.
3193,3175, 3216 , 3250, 3081,3033, 3081, 3033, N-C Stretch
1664,1482 , 1387. C-C Stretch 1677 ,1461, 1686 ,1516,1413,
1686, 1664 ,1550 ,1387 ,1177 ,1004 , N-C Stretch 1305 ,1225, C-C Stretch 1640, 1640,1567, 1664 , 1334 1295 , 1413,
1461,1305 ,O-C Stretch 1516,1295,1115, 841, 1072,995, 983, C-C-C Bending 855, 610, C-O-R Bending 1072, 983,746, H-O-C Bending 1305,1225, 1213, 1177, H-C-N Bending
1431,1334, H-C-C Bending 1664,1225,1213,1207, H-C-C Bending 1334,1207, 1461,1305,1225,1153, 1516,1251, 1213, C-N-C Bending 486, 417, C-C-C Bending 704,506, C-C-N Bending 1004, Bending O-C-C 506.
Peaks m/z: 69.12 (C5 H11 ), 81.88 (C5 H5 O), 94.34(C6 H6 O C5 H5 O), 108.4, 121.13 (C8 H9 O), 136.32, 149.17 (dialkyl phthalates), 159.20 (C10 H10 O), 176.75, 196.17,
206.66,239.41, 249.75, 271.03, 287.29,299.16.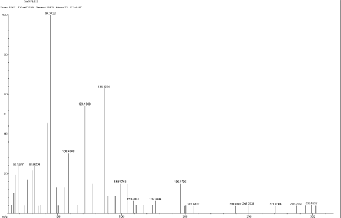
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 660
ISSN 2229-5518
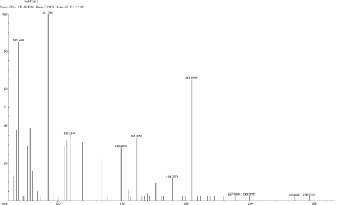
5.3 Scan1967, TIC=376240, Base: 4.4%FS, #ions=65, RT=10.88, m/z: 69.14(C5 H9 ), 92.12 (C6 H6 N), 119.21, 148.84 (dialkyl phthalates), 161.61 (C10 H10 NO),
188.93(C11 H21 NO2 ), 204.24, 262.57, 284.63.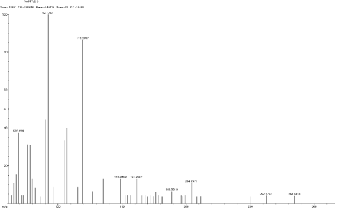
5.4 Scan 2095, TIC=387856, Base: 2.7%FS, #ions=61, RT=11.53, m/z: 69.14(C5 H9 ), 92.23 (C6 H6 N), 119.41,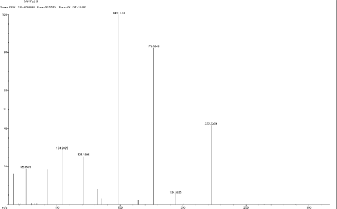
5.6 Scan 2392, TIC=4769040, Base 9.5%FS, #ions=72, RT=13.02, m/z: 75.35, 104.28, 121.19 (C9H9O), 149.11 (dialkyl phthalates), 176.53, 194.30 (C11 H21 NO2 ), 222.32.
ER
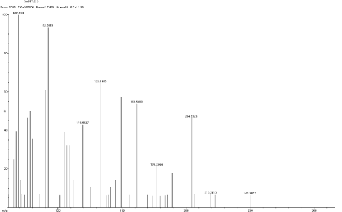
133.14, 161.54 (C10 H10 NO), 177.28, 204.28,219. 24,249.90.
5.5 Scan 2154, TIC=684016, Base 7.2%FS, #ions=62, RT=11.82, m/z: 62.12, 92.15(C6 H6 N), 109.28, 149.28(dialkyl phthalates), 161.35 (C10 H10 NO), 189.35(C11 H21 NO 2 ), 204.30,
237.98, 249.31, 285.0, 293.23.
5.7 Scan 2469, TIC=702080, Base: 6.2%FS, #ions=71, RT=13.41, m/z: 78.08(C5 H4 N some pyridines), 105.25,
119.20, 147.45, 161.40 (C10 H10 NO), 204.09, 222.41, 164.32.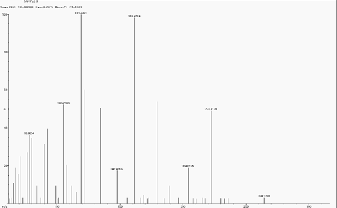
5.8 Scan 2470, TIC=629280, Base: 5.1%FS, #ions=78, RT=13.41 m/z: 80.0(C5 H5 O Pyrroles), 92.08(C6 H6 N),
105.27, 119.14, 134.2, 147.45, 161.37(C10 H10 NO), 167.42,
179.19, 189.02 (C11 H21 NO2 ), 204.31, 222.53, 236.61, 247.18,
268.77, 305.25.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 661
ISSN 2229-5518
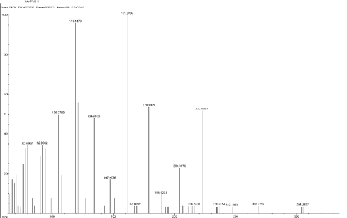
N and O), 101.20, 115.15, 129.32, 143.55, 157.49, 171.17,
185.23, 199.18, 213.26, 227.51, 239.62, 255.42, 264.60.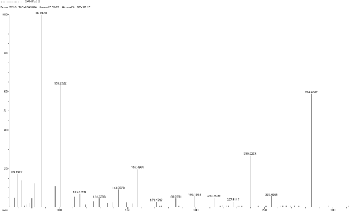
5.9 Scan 2906, TIC=370272, Base: 4.1%FS, #ions=59, RT=15.61. m/z: 76.25, 89.84 (C7 H5 Heterocyclics containing N and O), 105.17, 119.22, 129.14, 149.29 (dialkyl phthalate),
157.60, 171.39, 185.34, 200.28, 218.28, 228.73, 258.14,
290.94,305.
198.83, 212.46, 241.39, 259.45, and 281.87.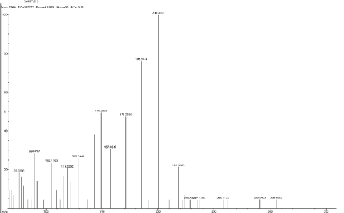
IJSER
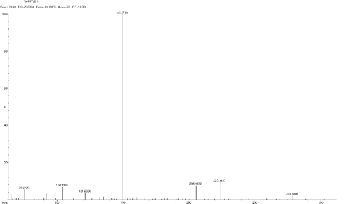
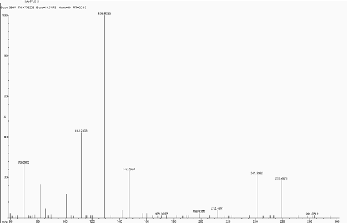
5.12 Scan 3216, TIC=1545856, Base 27.5%FS, #ions=76, RT=17.17, m/z: 69.19, 86.89(C7 H5 Heterocyclics containing
5.13 Scan 4220, TIC=437568, Base 10.2%FS, #ions=78, RT=22.22, m/z: 70.01 (C5 H10 Hydrocarbon), 82.02, 104.16,
131.87, 149.29 (Dialkyl phthalate), 167.24, 193.76 (C11 H21 NO2 ), 215.56, 240.83, 261.58, 279.42, 295.56.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 662
ISSN 2229-5518
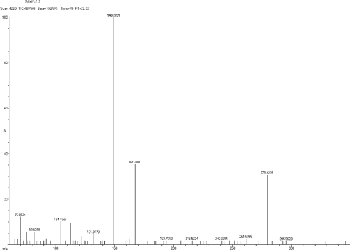
18, 19-Dimethoxy-6.8-diolxa-i-azapentacyclohenicosa -1, 3, 5,
10, 13, 15, 17, 19- octane and the molecular formula is
C20 H18 NO 4 .
I would express my sincere thanks to Mr. Govindasamy, Dr. Janarthanan and Mr. Shanmugam for their valuable suggestions, motivation and help for the betterment of my original research manuscript preparations.
17.17, 20.83, 22.22.
[1] B Duraiswamy, sagar Kumar Mishra, V Subhashini, SA Dhanraj, B Suresh. Indian Journal of Pharmaceutical science, 389- 391, 3(68), 2006.
[2] TR Suess, FR Stermitz- Journal of Natural products, ACS Publicaton, 1981.[3] Sastri, B.N., In; The wealth of India, A Dictionary of Indian Raw Materials and Industrial Products, Raw materials Vol VI; L-M, Publication and Information Directorate, CSIR, New Delhi. 226,1962.
[4] RK Radha, AM Varghese, S Seeni, J Thongchan, Science asia.org,2013.
[5] JW Huffman, EG Miller- The Journal of Organic
Chemistry,ACS Publication,1960.
[6] Raghunathan K,Ramdas, V.N.K., In; Tribal pockets of
Nilgiris, Recording of the field study on edicinal
flora and Health Practices. 2nd Edn, Central Council for research in India-Medicin and Homoeopathy,102,1978.
[7] S Rajan and M Sethuraman, Ancient Science of life. 1&2,
242-244, XII,1992.
[8] D Bajpai, PS Vankar, Fibers and Polymers, Springer,2007. [9] Duraiswamy B, Mishra SK, Subhashini V, Dhanraj SA,
Suresh B. www. ijpsonline.com /text.asp?2006/63 /386 /
26671.
[10] R Chatterjee, MP Guha-Journal of the American1951- wiley Online Library.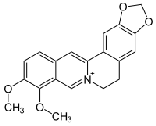
The berberine alkaloid isolated at 600C from leaf of the Mahonia leschenaultii plant. The experimental values of IR, UV and MS are used to predict the molecular structure, atomic stretching, possible molecular functional group, etc., for the confirmation of berberine alkaloid present in the Mahonia leschenaultii plant and are confirmed. The theoretical values are good agreement with the experimental values. The molecular structure is found by Gaussian software (V3) and also IUPAC name by ChemDoodle software and is represented as.
6.1Molecular structure of berberine alkaloid
[11] KV Tushar, S George, AB Remashree, Journal of plant, docsdrive.com.
[12] Fyson, P.F., In; The Flora of the Nilgiris and Pulney Hill Tops, Vol. I, I Edn., Bishen Singh Mahendra Pal Singh, Dehra Dun,1974, 14.
[13] Gamble, J.S., In; Flora of Presidency of Madras, Vol. I, Bishen Singh Mahendra Pal Singh, Dehra Dun,
1984, 32.
[14] P Muthumani, R Meera, P Devi, SA Mohamed Sheik Arabath, LV Seshu kumar Koduri, Sivaram Manavarthi. International Journal of Drug Formulation & Research-2010.
[15] D Yan, YM Han, L Wei, XH Xiao- joutnal of thermal analysis and. Springer,2009.
[16] Anupam Maurya, Santhosh kumar, Srivastava. Journal of
Chromatography. B- Elsevier (12)2008. [17] T.Ohnishi, R S Gall, MC Mayer. Analytical
biochemistry. 1975 Elsevier.
[18] Sreevidya, Narasimhan, Melhrotra, Shanta.
Journal of AOAC international (86)2003.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-2014 663
ISSN 2229-5518
• Lawrence T.,
Research scholar, Department of Physics, Karpagam
University, Coimbatore,Tamilnadu, India. – 641021,
Email. .tlaran@rediffmail.com,
• Dr. S. Gunasekaran D.Sc.,
Former Registrar, Periyar University, Salem.
Head (Rtd), PG and Research Dept. of Physics, Pachayappa’s College, Chennai.
Dean, Research and Development, St. Peter’s
University, Avadi, Chennai, Tamilnadu, India –
600054.
Email. sethugunasekaran@rediffmail.com.
IJSER
IJSER © 2014 http://www.ijser.org