The research paper published by IJSER journal is about Hybrid Process (Pervaporation-Distillation): A Review 1
ISSN 2229-5518
Hybrid Process (Pervaporation-Distillation): A Review
Syed Akhlaq Ahmad, Sohail Rasool Lone
Abstract— Pervaporation is one of the most active areas in membrane research, and the pervaporation process has been shown to be an indispensable component for chemical separations. Pervaporation is a relatively new membrane separation process that has elements in common with reverse osmosis and membrane gas separation. It is very difficult to separate the azeotropic or constant boiling mixtures by the ordinary distillation. Hybrid process which basically couples two processes together plays an important role in separation of azeotropic or constant boiling mixtures. A hybrid process basically exploits the advantages of pervaporation and distillation, while the negative aspects are minimized. Hybrid systems of different types reduce energy expenditures, make separations that are otherwise difficult and/or improve the degree of separation.
Index Terms— Pervaporation, Distillation, Membrane, Hybrid Process.
—————————— ——————————
DURING the past decade, industrial membranes have established themselves as indispensable components of chemical processing industries. Membrane-based technology is currently regarded as the new frontier of the chemical engineering and has been widely used for purification, concentration, fractionation of fluid mixtures. Pervaporation is a relatively new membrane separation process that has some features in common with reverse osmosis and membrane gas separation.
In pervaporation, the liquid mixture to be separated(feed) is placed in contact with one side of membrane and the permeated(permeate) is removed as a low-pressure vapour from the other side. The permeate vapour can be condensed and collected or released as desired. The chemical potential gradient across the membrane is the driving force for the mass transport. The driving force can also be created by applying either a vacuum or an inert purge(normally air or steam) on the permeate side to maintain the permeate vapour pressure lower than the partial
pressure of the feed liquid. Though pervaporation is one of the most popular areas of current membrane research, the concept of pervaporation separation is not new. The phenomenon of pervaporation was first discovered by Kober (1917),who originated the term in a publication reporting selective permeation of water from aqueous solutions of albumin and toluene through collodion (cellulose nitrate) films. Compared to other membrane separation processes, pervaporation is in a far less advanced state. Pervaporation is a widely used membrane separation process for the separation
————————————————
![]() Sohail Rasool Lone, is presently Pursuing M. Tech. in Chemical
Sohail Rasool Lone, is presently Pursuing M. Tech. in Chemical
Engineering from Aligarh Muslim University, Aligarh, email:
lonesohail92@gmail.com.
of liquid mixtures. Pervaporation is most suitable for the separation of close boiling or azeotropic mixtures. One of the most frequent industrial application areas of pervaporation is dehydration of solvents that form an azeotrope with water e.g. ethanol, isopropanol, tetrahydro-furane. Due to it’s high selectivity and low operational cost, pervaporation can be a useful alternative to distillation. The understanding of the pervaporation separation is incomplete. It is thought at present by some researchers that the preferential sorption of a component in the membrane is the prerequisite to the preferential permeation of that component. Based on this idea, several approaches for the selection of membrane materials have been proposed. On the other hand, an ideal sorption of liquid in polymer is generally assumed to describe the mass transfer using the commonly accepted solution-diffusion model. This controversy affects the proper understanding of the pervaporation mechanism and appropriate selection of the membrane materials. For a membrane to be efficient for a specific separation, it is always desirable to have a membrane with good permeability and selectivity. However, the hydrodynamic conditions of the flow for the feed and sometimes for the permeate cannot be overlooked. Distillation is the most widely used technique to separate liquid mixtures. However, the distillation separation of mixtures with an azeotropic compostion or with components with low relative volatility or close boiling mixtures is energetically expensive and auxillary substances are usually required. A hybrid process exploits the advantages of the pervaporation and distillation, while the negative aspects are minimized. Several processes have been presented in the literature and are applied in the industry such as for the dehydration of alcohols, aprotic solvents, and esters, as well as for the removal of VOC’s(volatile organic compounds) from aqueous streams. The role and application of pervaporation in stand- alone applications and in hybrid processes can be expanded if the involved capital cost of the pervaporation unit is reduced. The stability of the pervaporation membranes, the concentration and temperature polarization and the
IJSER © 2012
The research paper published by IJSER journal is about Hybrid Process (Pervaporation-Distillation): A Review 2
ISSN 2229-5518
temperature drop that occurs in the liquid are the factors that increase the required membrane area, the amount of auxillary equipment and the relating capital and operating cost. That is why hybrid pervaporation-distillation process is used, which exploits the advantages of pervaporation and distillation, while the negative aspects are minimized.
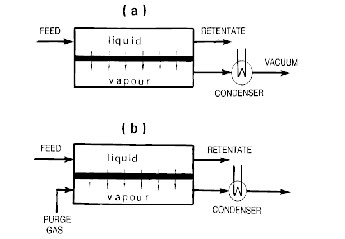
Pervaporation,in it’s simplest form is an energy efficient combination of permeation and evaporation. It is considered as an attractive alternative to other separation methods for a variety of processes. For example, with low temperatures and pressures involved in pervaporation, it often has cost and performance advantages for the separation of constant-boiling azeotropes. Pervaporation is also used for the dehydration of organic solvents and the removal of organics from aqueous streams. Additionally, pervaporation has emerged as a good choice for the separation of heat sensitive products. Pervaporation involves the separation of two or more components across a membrane by differing rates of diffusion through a thin polymer and an evaporative phase change comparable to a simple flash step.
Fig. 1. Schematic Diagram of the Pervaporation Process. (a) Vacuum Pervaporation, (b) Purge gas Pervaporation.
Pervaporation can be used for breaking azeotropes,dehydration of solvents and other volatile organics,organic/organic separations such as ethanol or methanol removal,and waste water purification.
Pervaporation separation is governed by the chemical nature of the macromolecules that comprise the membrane, the physical structure of the membrane, the physico-chemical properties of the mixtures to be separated, and the permeant- permeant and permeant-membrane interactions. Pervaporation transport is usually described to be a three-step process: solution-diffusion-evaporation. The separation is based on the selective solution and diffusion, i.e., the physical-
chemical interactions between the membrane material and the permeating molecules, not the relative volatility as in the distillation. Therefore, pervaporation is commonly considered to be profitable for the separation of azeotropic and close- boiling mixtures, which requires at present the use of energy- intensive process. Pervaporation can be operated at low feed pressures and at ambient temperature or even below this, and no additional chemicals are required for the separation. Unlike reverse osmosis, pervaporation transport is not limited by the osmotic pressure, because the driving force for the mass transfer through the membrane is provided by lowering the chemical potential of the permeate stream on the downstream side, and consequently, the feed pressure is not critical. Unike the reverse osmosis and membrane gas separation, pervaporation involves a phase change of permeating species from the liquid to the vapour-state. Consequently, energy is needed for the vaporization of the permeate. Thus, from an energy consumption point of view, pervaporation is especially promising when the concentration of the preferentially permeating species in the feed is low. The heat that is required for this change of phase from the liquid to vapour can be supplied either in the feed liquid or by a sweeping fluid on the permeate side or directly to the membrane. In pervaporation, selectivity is independent of the vapour-liquid equilibrium, which is of interest especially in case of azeotropic points and/or small boiling curve-differences in the components. Conventional separation techniques require either the addition(and subsequent separation) of a suitable solvent or a pressure variation. In both cases, at least one additional column is required, because of the separation problem. In addition, the specific energy consumption is higher compared with simple distillation. In pervaporation, the vapour-liquid equilibrium is so perturbed by the introduction of a selective membrane that azeotropic mixtures can be efficiently fractionated. However, the pervaporation is expensive, because of the relatively low permeate fluxes and the usually low condensation temperatures, and a combination of distillation/pervaporation is often superior.
Pervaporation is a rate-controlled separation process. In developing pervaporation membranes, three important aspects must be well addressed: (i) membrane productivity, (ii) membrane selectivity, and (iii) membrane stability. Membrane productivity is a measure of the quantity of a component that permeates through a specific area of membrane surface in a given unit of time. Membrane productivity is frequently characterised by permeation flux , J , which relates the product rate to the membrane area needed to achieve the separation. Permeation flux depends on both the intrinsic permeability and the effective thickness of the membrane. The commercialization of the pervaporation technique is, to a large extent, being attributed to the engineering approach of making thin membranes in asymmetric and composite forms.
IJSER © 2012
The research paper published by IJSER journal is about Hybrid Process (Pervaporation-Distillation): A Review 3
ISSN 2229-5518
When describing the selectivity of a membrane for the separation of a mixture composed of components A and B, the separation factor is defined as:
diffusivity. However, these equations cannot be taken for granted unless they are used within the experimentally established range for which the relationships expressed for
(Y / 1
Y )(1
X / X )
![]()
(1)
diffusion and thermodynamic equilibria are applicable. The![]()
![]()
![]()
![]()
where X and Y are the molar fractions of the more permeable component A in the feed and permeate respectively. The numerical value of is independent of the concentration units used, as being the ratio of ratios. When the separation factor is unity, no separation occurs; when it approaches infinity, the membrane becomes perfectly “semipermeable”. It is the membrane selectivity that forms the basis for the separation of a mixture. It should be noted that only when the concentration polarization is negligible will the selectivity as expressed by equation (1) be an intrinsic property of the membrane. Otherwise the feed concentration on the membrane surface has to be subistituted for X . Generally, membrane permeability and selectivity have to be determined experimentally. Also sometimes, membrane selectivity is expressed in terms of the enrichment factor , which is simply defined as the ratio of concentrations of preferentially permeating species in the permeate and in the feed. Unlike , the numerical value of depends on the concentration units used. is more significant term than from the physico-chemical point of view, although the term is sometimes more convenient to use especially when dealing with very dilute feed solutions.
Membrane stability is the ability of a membrane to maintain both the permeability and selectivity under specific system conditions for an extended period of time. Membrane stability is affected by the chemical, mechanical, and thermal properties of the membrane. When considering polymeric membranes for the separation of anhydrous organic mixtures, membrane stability is of prime importance.
A proper understanding of the membrane separation mechanism may provide direct information on the research and development of an appropriate membrane. Because of the complicated permeate-membrane interactions, it is difficult to formulate a single explanation for the complex transport process. There are principally two approaches to describe mass transport in pervaporation:(i) the solution-diffusion model and (ii) the pore- flow model.
The solution-diffusion model is accepted by the majority of researchers. According to this mechanism, pervaporation consists of three consecutive steps:(i) sorption of the permeant from the feed liquid to membrane,(ii) diffusion of the permeant in the membrane ,and(iii)desorption of the permeant to the vapour phase on the downstream side of the membrane(see Figure 2).In general, solubility and diffusivity are concentration dependent. A number of mathematical equations for mass transport have been formulated on the basis of Fick’s diffusion equation using different empirical expressions of concentration dependence of solubility and/or
transport of a single component through a non-porous
homogeneous membrane has been relatively well described.
The concentration dependence of diffusivity is often expressed
by exponential or linear forms. Assuming thermodynamic
equilibria exists at both membrane interfaces, the steady-state
flux equation can be readily derived on the basis of Fick’s law
for one-dimensional diffusion normal to membrane surface.
The difference between the solution-diffusion and pore-flow
mechanisms lies in the relative size of pores and the
permeance through it. For membranes, the transport is best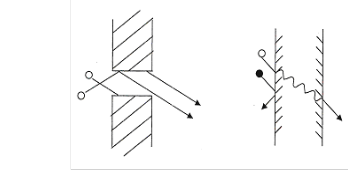
described by the solution-diffusion model and Fick’s law.
Fig. 2. Showing Pore-Flow and Solution-Diffusion Model respectively.
In distillation (fractionation), a feed mixture of two or more components is separated into two or more products, including, and often limited to an overhead distillate and a bottoms, whose composition differ from that of the feed. Most often, the feed is a liquid or a vapour-liquid mixture. The bottoms product is almost always a liquid, but the distillate may be a liquid or a vapour or both. The separation requires that(i) a second phase be formed so that both liquid and vapour phases are present and contact each other on each stage within a separation column, (ii) the components have different relative volatilities so that they will partition between the two phases to different extents, and(iii) the two phases can be separated by gravity or other mechanical means. Distillation differs from absorption and stripping in that the second fluid phase is usually created by thermal means(vaporization and condensation) rather than by the introduction of a second phase that may contain an additional component or components not present in the feed mixture.
Distillation is a well known technique with lower capital cost than pervaporation. However, the energy consumption in pervaporation is lower, because it is required only for the vaporization and expansion of compounds that are selectively transported through the membrane. Capital cost in pervaporation is high due to the cost of the membranes, the modules and the auxiliary equipment. Distillation separation
IJSER © 2012
The research paper published by IJSER journal is about Hybrid Process (Pervaporation-Distillation): A Review 4
ISSN 2229-5518
of mixtures with an azeotropic composition or with components with low relative volatility or close boiling mixtures is energetically expensive and auxiliary substances are required. That is why hybrid process (Pervaporation- Distillation) is used for the separation of azeotropic mixtures or close boiling mixtures. A hybrid process exploits the advantages of pervaporation and distillation, while the negative aspects are minimized.
It has been widely recognized that membrane separation processes can offer many advantages over conventional mass transfer processes. A large number of membrane separation processes are currently being practised in various sectors of industries. Despite the advantages, membrane processes often suffer from shortcomings when used individually. To overcome such limitations, membrane-based hybrid processes have been developed to maximize the productivity of the target separation processes. Membrane technologies have recently emerged as an additional category of separation processes to the well-established mass transport processes. Membrane separation technologies offer advantages over existing mass transfer processes like high selectivity, low energy consumption, moderate cost to performance ratio and compact and modular design.
Although conventional separation processes still receive a great deal of attention in literature, other efficient, though expensive, separation processes are increasingly being considered as alternatives. There are several reasons for this newly arising interest, the most relevant being environmental concern and improvement in efficiency of separation.
During the past decade, hybrid distillation/pervaporation processes have been developed for the separation of azeotropic mixtures as well as the dehydration of aqueous- organic mixtures using hydrophilic or organophilic membranes according to the separation task to be accomplished. When pervaporation modules are used alone, they are more expensive than pervaporation membranes combined with a conventional process, thus the interest in using them in the context of a hybrid process. One of the applications is the separation of azeotropic mixtures, for which they are especially suited, because the separation does not depend on the equilibrium between components, but on the difference between the activities on the two sides of the membrane. The drawback for using membrane processes is that only low concentrations and low flow rates of feed streams can be treated to keep the prices reasonably low. Hybrid processes provide an interesting alternative, because using a conventional separation step such as distillation for the initial separation task leaves the last difficult separation to the membrane, reducing the concentrations and flow rates to be treated, thus requiring a less expensive process.
However, membrane processes have several inherent limitations. For example, a membrane system designed to treat waste water may be limited by the water’s osmotic pressure, viscosity, temperature, and high concentration of suspended solids. Therefore, the optimal separation process in many cases may be a “Membrane-based hybrid process” that combines either a membrane process with a conventional process or a membrane process with other membrane process. A hybrid process is appropriate when it offers significant advantages(such as lower capital and production costs or reduced energy requirements) over the exclusive use of conventional processes. Moreover, membrane hybrid processes may achieve separations that are otherwise impractical or altogether impossible to achieve with either conventional process. Hybrid process integrating pervaporation with distillation can have various configurations which are shown in Figure 3.
Fig. 3. Basic Configurations for Hybrid (Pervaporation-Distillation) Processes.
More recently hybrid processes integrating pervaporation with other variable liquid separating technologies are gaining momentum. With these developments, we have more reasons to believe that pervaporation will play even more important roles in future.
Hybrid processes like combination of distillation and pervaporation are very promising especially in cases where high product purities are required.
To reduce costs, particularly energy costs, make possible a difficult separation, and/or improve the degree of separation,
IJSER © 2012
The research paper published by IJSER journal is about Hybrid Process (Pervaporation-Distillation): A Review 5
ISSN 2229-5518
hybrid systems, consisting of two or more separation operations of different types in series are used.
Hybrid systems of different types reduce energy expenditures, make separations that are not otherwise possible and/or improve the degree of separation.
It is now widely been recognized that the membrane separation processes can offer many advantages over conventional mass transfer processes. A large number of membrane separation processes are currently being practised in various sectors of industries. Despite the advantages, membrane processes often suffer shortcomings when used individually. To overcome such limitations, membrane-based hybrid processes have been developed to maximise the productivity of the target separation processes. A hybrid process tries to exploit the advantages of both the processes, while minimizing the negative aspects. Pervaporation is an emerging membrane separation process. More recently, the hybrid processes integrating pervaporation with other variable liquid separating technologies are gaining momentum. With these developments we have more reasons to believe that hybrid processes will play even more important roles in future.
This work was supported by The Aligarh Muslim University
(AMU),Aligarh.
[1] Beartiz Gonzalez Gonzalez and Inmaculada Ortiz Uribe, “Mathetical Modeling of the Pervaporative Separation Methanol-Methyl-tert butyl ether Mixtures,Ind. Eng. Chem.,40,1720-1731(2001).
[2] Beartiz Gonzalez & Inmaculada Ortiz, “Modeling and Simulation of a hybrid process (pervaporation-distillation) for the separation of azeotropic mixtures of alcohol-ether, Journal of Chemical Technology and Biotechnology, 77, 29-42(2001).
[3] Carsten Buchaly,Peter Kreis,Andrzej Gorak, “Hybrid Separation Processes-Combination of Reactive Distillation with Membrane Separation,Proceedings of the European Congress of Chemical Engineering (ECCE-6),Copenhagen,16-20 September,(2007).
[4] Daniel Eumine Suk,Takeshi Matsuura, “Membrane –Based Hybrid
Processes:A Review”,Separation Science and Technology,41:4,595-
626(2006).
[5] Frank Lipnizki, Robert W. Field & Po-Kiong Ten, “ Pervaporation- based Hybrid Processes:A Review of Process Design,Applications and Economics”,Journal of Membrane Science,153,183-210(1999).
[6] Javier Fontalvo Alzate, “Design and Performance of Two-Phase Flow Pervaporation and Hybrid Distillation Processes”, PhD Thesis, Technische Universiteit Eindhoven (2006).
[7] J.G. Wijmans &R.W. Baker, “The Solution-Diffusion Model:A
Review”,Journal of Membrane Science,107,1-21(1995).
[8] J.D. Seader & Ernest J. Henley, “Separation Process Principles”, 2 nd
Edition, Wiley India Pvt.Limited,2010.
[9] Maria C. Daviou, Patricia M. Hoch, Ana M. Eliceche, “Design of Membrane Modules used in Hybrid Distillation/PervaporationSystems”,Ind.Eng.Chem.,43, 3403-
3412,(2004).
[10] Mohammed Z. Mohammed, “Mathematical Modeling and Simulation for Production of MTBE By Reactive Distillation”,M.Tech. Thesis, University of Technology, (2006).
[11] Uwe Hommerich, Robert Rautenbach, “Design and optimization of combined Pervaporation /Distillation processes for the production of MTBE”, Journal of Membrane Science,146,53-64,(1998).
[12] Wolfgang Stephan, Richard D. Noble, Carl A. Koval, “Design methodology for a Membrane/Distillation Column Hybrid process”, Journal of Membrane Science, 99 , 259-272(1995).
[13] Xianshe Feng & Robert Y.M. Huang, “Liquid Separation by
Membrane Pervaporation:A Review”, Ind.Eng.Chem,36,1048-
1066,(1997).
IJSER © 2012