International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1234
ISSN 2229-5518
Electroanalytical Lab, Department of Chemistry, Sri Venkateswara University
Tirupati-517502, Andhra Pradesh, India
Polyaniline (PANI) coatings were electrodeposited on the surfaces of glassy carbon electrodes (GCEs) to form new electrodes, i.e. PANI/GCEs. An electrocatalytic method has been proposed for determining dithiocarbamate-based pesticide Mancozeb using a Glassy carbon electrode modified with polyaniline. The electrochemical reduction behaviour of mancozeb fungicide at bare glassy carbon electrode and glassy carbon electrode modified with polyaniline was studied in aqueous solutions by Differential Pulse Voltammetry (DPV), Cyclic Voltammetry (CV) and Linear Sweep Voltammetry (LSV). A linear response over a
mancozeb concentration of 0.01M to 4.5×10-7 M was exhibited with detection limits (S/N=3)
of 2.6 ×10-8 M. The high sensitivity and selectivity of those electrodes were demonstrated by its practical application to the determinations of trace amounts of mancozeb in milk and serum samples.
Ethylene bisdithiocarbamate (EBDC) base pesticides (including Nabam, Mancozeb, Ferbam, Maneb, and Zineb) have been widely used as broad-spectrum fungicide, bactericide, and algaecide. These have been widely used to control algae in rice field as well as the fungal diseases of fruits, vegetables, paddy and ornamental plants [1, 2]. The active ingredient, Mancozeb [(manganese ethylene bis(dithiocarbamate) (polymeric) complex with zinc salt)], is a contact fungicide in a subclass of carbamate pesticides called dithiocarbamates (DTCs). DTCs are applied in agriculture as pesticide and in the rubber industry as vulcanization accelerators and anti-oxidants. Zineb (Zinc (II) ethylenebisdithiocarbamate) and Maneb (Manganese (II) ethylenebisdithiocarbamate) are the important dithiocarbamate fungicides
[3-6]. Mancozeb is a similar fungicide like maneb, but it is the combination of zinc and
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1235
ISSN 2229-5518
manganese metal chelating agents. DTCs also a chelating agent with various metal ions, such as Fe+2, Mn+2, Cu+2, Na+, Zn+2 and Ni+2 to form coordination complexes and to act as a fungicide. The toxicity is enhanced significantly if metal complex is formed. Thus, a highly sensitive tool is essential for further investigation of this class of metal dithiocarbamate compounds and is used for the treatment of carcinogenicity due to its advantages of low acute toxicity combined with strong activity, low cost and short environmental persistence, the use
of maneb and other EBDCs is increasing worldwide. DTCs do not belong to systemic fungicides but are protectant fungicide applied prior to fungus infection.
The analysis of alkylenebisdithiocarbamates of some bivalent metal ions is hampered by their low solubility, low stability and polymeric structure [7]. DTCs are mainly determined by indirect methods including spectrophotometry, gas chromatography or thin layer chromatography based on the reaction products, liberated after decomposition by hot mineral acids to amine and carbon disulphide (CS 2 ) [8,9]. It is important that these methods are typically unable to distinguish among various DTCs since most can be decomposed to carbon disulphide. DTCs have also been determined by UVvisible spectrophotometry [10,
11], voltammetry [12], chromatography techniques [13], fluorimetry (14), titrimetry (15), gas chromatography (16) and capillary electrophoresis (17). Other methods for the determination of DTCs rely on the measurement of the metallic portion of the compounds. Most of the methods mentioned above are time consuming and require pretreatment of samples such as extraction and preconcentration procedures. They also require a calibration with standard mancozeb solution. From the literature survey, no method has been described for the analysis of mancozeb at bare GCE and modified glassy carbon electrode with polyaniline, till now.
Recently, modifications of electrodes for detection of trace heavy metals by means of conductive polymers have received considerable attention due to their superior electrical conductivities, good adhesion properties and suitable structural characteristics [18,19]. Due to its facile preparation, high conductivity and good environmental stability [20], conductive polyaniline (PANI) can be electrochemically coated on the surfaces of glassy carbon electrodes (GCEs) and forms a porous coating [21, 22]. PANI coatings are stable and can remain intact for a long time as long as they are not mechanically damaged [23]. The microstructure of PANI coatings can be controlled by fabrication methods and conditions such as temperature, monomer concentration, deposition potential and time, which then greatly influences their electrical conductivities.[24] For example, PANI coatings prepared by
conventional potentiodynamic and potentiostatic methods were usually thick and compact
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1236
ISSN 2229-5518
[25]. A compact coating has a relatively small specific surface area and a poor electrical conductivity, which are unfavorable for the construction of an electrochemical sensor. Thus, PANI coatings formed by electrodeposition on GCEs have usually been via a method of cyclic voltammetry (CV). In this study, we have addressed the challenge to develop a rapid method for the specific determination and analysis of individual DTCs based on the use of voltammetry. In the present paper, we demonstrate the reduction behaviour of mancozeb by CV and recovery of mancozeb by DPV. The electrochemical behaviour of mancozeb has been discussed also by LSV and the enhanced sensitivity of differential pulse voltammetry is attributed.
Experiments were conducted with Autolab PGSTAT 101 supplied by Metrohm, The Netherlands. All electrochemical experiments such as polymerization of PANI and measurements were performed using an electrochemical workstation, having a conventional three-electrode cell configuration with a GCE or PANI/GCE of a diameter of 3 mm as the working electrode, saturated calomel electrode(SCE) as a reference electrode and platinum wire as a counter electrode. Electrochemical experiments were carried out in a 2-mL
voltammetric cell or a flow injection system at room temperature (250C). All potentials are
referred to the Ag/AgCl reference electrode. All potentials are quoted vs. SCE reference electrode. All pH measurements were made with the aid of a digital pH meter using a combined glass electrode.
Aniline monomers were freshly distilled under a reduced pressure and stored at a low temperature (-5°C) in a nitrogen atmosphere. Acetic acid and sodium acetate were used for the preparation of a 0.1 M acetate buffer solution. The working solutions were prepared by dilution from the stock solution. The stock solution of mancozeb was prepared by dissolving in 0.1N HCl and make up with doubly distilled water and stored at room temperature. Acetate buffer of the pH 4.5 was prepared by 982.3 ml of 0.1M acetic acid and 17.7 ml of 0.1 M
sodiumacetate (tri-hydrate). All the chemicals used were of analytical reagent grade (Merck).
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1237
ISSN 2229-5518
Thoroughly polished GCE surfaces using alumina slurry on a soft cloth were sonicated in first ethanol and then doubly distilled water for 3 min each to remove possible contaminants. The PANI coatings were formed on the GCE surfaces by dipping the polished GCEs and electrochemically deposited at a constant potential of 0.80 V for 120 s in an aqueous solution of 0.1 M LiClO4 and 0.1 M carbonate containing 0.15 M polyaniline as well as in a 0.25M H2 SO4 electrolyte containing 7.3 mM aniline monomers via a CV process from
−0.2 to 0.9 V at a scan rate of 50 mV/s for cycles under a nitrogen environment. After the
polymerization of PANI [26], the fabricated PANI/GCEs were dipped into doubly distilled water for 3 min to remove unpolymerized aniline monomers remaining in the PANI coatings if any. After each polishing, the electrode was sonicated in ethanol and doubly distilled water for 5 min, successively, in order to remove any adsorbed substances on the electrode surface. Finally, it was dried under nitrogen atmosphere ready for use. The electrode was then transferred into 0.1 M HClO4 solution for 12 h aging. The polyaniline modified electrod was denoted as PANI/GCE.
The analytical characteristics of the prepared GCE modified electrode were subsequently evaluated under optimum conditions. The electrochemical techniques have led to the advancement in the field of analysis because of their sensitivity, low cost and relatively short analysis time, as compared with other techniques. Electrochemical techniques have proven to be useful for development of very sensitive and selective methods for the determination of organic molecules including pesticides. In addition application of electro analytical techniques include the determination of electrode mechanisms. The reductive process of mancozeb involving four electrons and four protons will cause the addition of four protons to the thio-groups. Also, the geometric structure of mancozeb will be changed when the reductive process occurs. Both the carbon disulfide groups will be distanced from each
other and loss the capability of chelating to the Zn+2 and Mn+2 ions. In consequence the Zn+2
and Mn+2 ions will be free from the carbon disulfide groups to the bulk solution [27].
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1238

ISSN 2229-5518
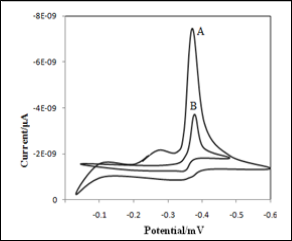
We first compared the signal of mancozeb with bare GCE and also modified GCE. Fig.1 depicts the cyclic voltammetric responses for electrochemical reduction of mancozeb in acetate buffer of pH 4.5, recorded at scan rate of 100 mVs-1. The cyclic voltammograms for the GCE and GCE-PAN in a solution of mancozeb in 0.1 M acetate buffer at pH 4.5 are shown in Fig.1. Cyclic voltammetric measurements were carried out with 4.5×10-7 M mancozeb at GCE/GCE-PAN. Mancozeb showed irreversible behaviour with a reduction peak at -0.41 V for the bare electrode and however reduction peak current increased at same potential for the modified electrode while no detectable signal was observed. Cyclic voltammogram of mancozeb generated small reduction current at GCE. At GCE-PAN exhibited much larger reduction current (well-resolved peak) due to the excellent electrical
conductivity of polyaniline.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1239
ISSN 2229-5518
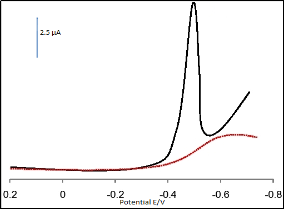
Linear scan voltammetry generally has a much lower sensitivity as compared with DPSV, but it is often used to investigate the electrochemical behaviour of electrochemical systems. Fig.2 depicts linear sweep voltammograms of mancozeb on the bare GCE and on polyaniline modified GCE in pH 4.5 acetate buffer. It is apparent that electrochemical reduction of mancozeb at the bare GCE proceeds very slowly and almost no current response is observed. An enhanced peak for mancozeb reduction is observed when the polyaniline modified GCE is used, the peak potential is located at approximately -0.43 V (relative to the SCE)
.Based on the above voltammetric response, the mechanism of reduction behaviour of
mancozeb was proposed in scheme 1 [28].
IJSER © 2013 http://www.ijser.org
International JournEaml opfeSrciiceanltiFfiocr&mEunlagineering Research, Volume 4, Issue 7, July-2013 1240
ISSN 2229-5518
![]() SCSNHCH2CH2NHCSHSMn
SCSNHCH2CH2NHCSHSMn ![]()
Chemical Structure
(Zn)y
x
![]()
![]()
S
![]()
![]()
-S C
![]()
N H
H2
![]()
![]()
C CH2 N
![]()
![]()
![]()
S
![]()
![]()
![]()
![]()
![]()
C S Mn S C
![]()
H x
![]()
![]()
![]()
S
![]()
![]()
N Zn
NH S C
S y
Reduction Mechanism in Acetate Buffer media
![]()
![]()
![]()
![]()
![]()
![]()
S NH C
S
[Mn+2]
[Zn+2]y
pH 4.5
![]()

S
![]()
NH C SH
SH
![]()
+ Mn+2 + Zn
![]()
NH C S
S
Acetate Buffer
4H+, 4e-
x
![]()
![]()
NH C
S x+y
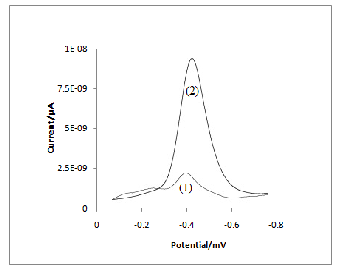
In two cases, one reduction peak was observed, but the reduction signals are different. The voltammogram recorded at PAN/GCE (curve 2) is best developed with most satisfactory signal to noise characteristics compared to GCE (curve 1). It can be noticed that the maximum of reduction peak of model compound is of about -0.4V at both electrodes
(relatively close). In fig.3, by carrying out with DPV for 4.5×10-7M mancozeb at the bare
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1241
ISSN 2229-5518
GCE and the modified GCE, the difference is clearly demonstrated. The observed voltammetric behaviour is typical for electrochemically generated electroactive mancozeb.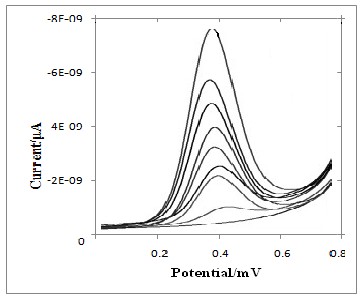
Fig.4 displayed the differential pulse voltammetric response of mancozeb at GCE/PAN. Well resolved peaks proportional to the concentration of corresponding mancozeb were observed in the range of 0.01M to 4.5×10-7M. The linear regression equation was /(μA) = 1.55+ 0.0884, with a correlation coefficient of 0.0991 (calibration curve is not shown). The peak current increased linearly with an increase in concentration. In a similar manner, DPV studies of mancozeb at bare GCE and modified systems were carried out.
Recovery and relative standard deviations were calculated and the results are presented in
Table 1.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1242
ISSN 2229-5518
Sample | Added (M) | Found (M) | Recovery* (%) | RSD(%) | |
Milk | 1 | 0.400 | 0.385 | 96.3 | 1.50 |
Milk | 2 | 0.600 | 0.571 | 95.2 | 1.42 |
Milk | 3 | 0.800 | 0.792 | 99.0 | 1.63 |
Serum | 1 | 0.400 | 0.395 | 98.8 | 1.10 |
Serum | 2 | 0.600 | 0.607 | 92.2 | 1.30 |
Serum | 3 | 0.800 | 0.806 | 97.8 | 1.40 |
To further demonstrate the practicality of the proposed method, the recovery test was studied
by adding different amounts of mancozeb into milk and serum samples.The recoveries were
from 95.0% to 99.0%. The results indicated that the proposed method was highly accurate,
precise and reproducible. It can be used for direct analysis of relevant samples.
8E-09
6E-09
4E-09
2E-09
0
0 2 4 6 8 10
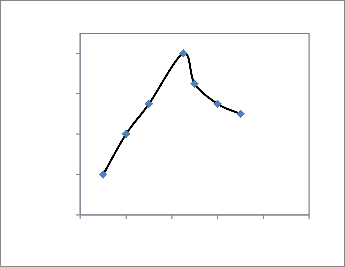
The influence of solution pH on the response of 4.5× 10-7M mancozeb was examined over the pH range 0.0–8.0 in acetate buffer solution by CV at a scan rate of 100 mV s−1. The acidity of the detection medium is a key parameter that can affect the mass transport to the electrode surface, especially when the redox process involves some protons as in the present
case. The electrode response was low for the most acidic media (pH 1–3). At pH values
above 3, the electrode signal was found to grow up markedly to reach the highest value at pH
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1243
ISSN 2229-5518
4.5, and then decreased strongly at pH 5 down. A solution with pH around 4.5 was selected as the optimal situation for the detection of the pesticide and used for further experiments. It is concluded that the voltammetric behaviour is controlled by the amount of electro active species adsorbed on the modified electrode, which is influenced by the solution pH. The pH of the detection solution was one of the most important issues to be considered in the measurement of the voltammetric response. We observed that the stripping signal increased with an increase of pH up to 4.5 and then decreased at higher pH, which might be due to the degradation of mancozeb in basic media. To achieve the highest signal, pH 4.5 acetate buffer was used in the measurements.
The stability of PAN-GCE electrode was examined in experiment. The modified electrode was stored in the 0.1 mol L−1 acetate buffer solution (pH 4.5) after every experiment. The cyclic voltammetric experiments were carried out using modified electrodes once a day at the same operation conditions. Calibration curves can hardly change by means of long times. Then, it showed that the electrochemical sensor has more stability, with relative standard deviation .
Polyaniline (PANI) is one of the most extensively used conjugated polymers in the design of electrochemical sensors. A novel electrochemical sensor for determination of determination of mancozeb was fabricated by electrodeposition step. The constructed PANI/GCE exhibited a strongly electrocatalytic activity toward the reduction of mancozeb. The modified electrode was used for sensitive determination of mancozeb using DPV technique. PANI coatings were electrodeposited on glassy carbon electrodes to form PANI/GCE electrodes. This work reveal that pulse voltammetric techniques are particularly designed for measuring very low levels of pesticides in the environmental and biological samples, mainly when they are used in connection with modified electrodes. The lower
detection limit of mancozeb was estimated to be 2.6×10-8M. The PANI/GCE had a good
stability, reproducibility and anti-interference ability. Since DPV has higher sensitivity and better resolution than CV, DPV was used for the determination of mancozeb present in biological samples such as serum and milk. Taking into account all the above, we can
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1244
ISSN 2229-5518
conclude that the DPV using modified GCE electrode can be a fast and low-cost tool for the detection and determination of selected pesticide in the presence of the dissolved oxygen. Further improvement of the sensitivity of the method can be achieved by the preconcentration of the sample.
I am very much thankful to UGC-RGNF supporting for financial assistance.
[1]. Garcinuno RM, Ramos L, Fernandez-Hernando P, Camara C. 2004a. J Chromatogr A.
1041:35–41.
[2]. Garcinuno RM, Fernandez-Hernando P, Camara C. 2004b. J Chromatogr A.
1043:225–229.
[3]. Metha S K, Malik A K, Rao A L J, (2004). Electron J Agric Food Chem 3: 784. [4]. Weissmahr K W, (1998),Anal Chem 70: 4800.
[5]. Turker A R, Sezer B, (2005), G U J Sci 18:93. [6]. Kesari, R, Gupta V K, (1998), Talanta 45: 1097.
[7]. Bardarov, V. and Zaikov, C., J Chromatogr., 479: 97-105 (1989).
[8]. Rao, A.L.J., Malik, A.K. and Kapoor J., Talanta, 40: 201-203 (1993). [9]. Malik, A.K. and Rao, A.L.J., Talanta, 44: 177-183 (1997).
[10]. Kesari, R. and Gupta, V. K., Talanta, 45: 1097-1102 (1998).
[11]. Mathew, L., Rao, T.P., Iyer C.S.P. and Damodaran, A.D., Talanta, 42: 41-43 (1995). [12]. Ulakhovich, N.A., Medyantseva, E.D., Froleva, V.F. and Romanova, O.N., Zh. Anal.
Khim. 38: 1963-1968 (1983).
[13]. Gustafsson, K.H. and Fallgren, C.H., Agric. Food Chem., 31: 461-463 (1983).
[14]. Ruiz, T. P., Martinez, L. C., Thomas, V. and Casajus, R., Talanta, 43: 193-198 (1996).
[15]. Verna, B. C., Sidhu, H.S. and Sord, R.K., Talanta, 29: 703-705 (1982). [16]. Ponomarev A.S. and Shtykov S.N., J. Anal. Chem., 55: 47-51 (2000).
[17]. Malik, A.K. and Faubel, F., Fresenius J. Anal. Chem,. 367: 211-214 (2000).
[18]. M.D. Imisides, R. John, P.J. Riley, G.G. Wallace, Electroanalysis 3 (9) (1991) 879. [19]. W.W. Zhu, N.B. Li, H.Q. Luo, Anal. Lett. 39 (11) (2006) 2273.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 7, July-2013 1245
ISSN 2229-5518
[20]. Z.X. Wei, M.X. Wan, T. Lin, L.M. Dai, Adv. Mater. 15 (2) (2003) 136. [21]. S. das Neves, M.A. De Paoli, Synth. Met. 96/1 (1998) 49.
[22]. H.N. Dinh, J.F. Ding, S.J. Xia, V.I. Birss, J. Electroanal. Chem. 459 (1) (1998) 45. [23]. J.H. Santos, M.R. Smyth, R. Blanc, Anal. Commun. 35 (10) (1998) 345.
[24]. J.M. Ribo, M.C. Anglada, J.M. Hernandez, X.D. Zhang, N. Ferrer-Anglada, A.
Chaibi, B. Movaghar, Synth. Met. 97 (3) (1998) 229.
[25]. X.H. Gao, W.Z. Wei, L. Yang, M.L. Guo, Electroanalysis 18 (5) (2006) 485. [26]. J. Li, X.Q. Lin, Biosens. Bioelectron. 22 (2007) 2898– 2905.
[27]. Meng Shan Lin, Bor Iuan Jan, Hoang-Jyh Leu, Jhy Shing Lin, Analytica Chimica
Acta 388 (1999) 111-117
[28]. C.A. Martínez-Huitle et al. / Port. Electrochim. Acta 28 (2010) 39-49
IJSER © 2013 http://www.ijser.org