International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 542
ISSN 2229-5518
Effect of Forest road on tree regeneration
*Somayeh Karamirad1, Ehsan Abdi1 , Baris Majnounian1, Hormoz Sohrabi2
Iran.
![]()
![]()
Manuscript Info Abstract
![]()
Received: xxxxxxx
Final Accepted: xxxxxxxxxxxxxx
Published Online: xxxxxxxxxxxx
Tree regeneration
The distribution and abundance of species can be strongly influenced by habitat suitability. Road construction can change habitat suitability by affecting resources and microclimate. We examined the distribution of tree regeneration and their relationships with forest roads in a temperate forest, Hyrcanian zone, Iran. At six different distances from the verge of forest road (0, 5, 10, 25, 50 and 100 m), the species abundance and coverage were recorded. The results showed that similar to the regeneration rate, the species richness and abundance are clearly related to the distance from the roads. The effect of the road on species richness and diversity was observed up to
10 m from the road verge. As would be expected, the results revealed that the light demanding species such as Acer velutinum were more frequent in road verges compare to shade tolerant species such as Fagus orientalis. As a conclusion, minimizing road density to keep sections of forest area large enough to conserve diversity and also composition of forest species is necessary for sustainable forest resources.
Copy Right, IJAR, 2013,. All rights reserved.
![]()
Forest road development is a primary mechanism of fragmentation, removing original land cover, creating edge habitat, altering landscape structure and function, and increasing human access (Forman and Alexander, 1998; Spellerberg, 1998; Trombulak and Frissell, 2000). Roads create linear gaps that remove forest area, make micro and meso-climatic changes through variation of the received sun radiation, wind regimes, moisture and temperature (Forman et al., 2002). High light and disturbance levels due to canopy openness are widely known to impact the vegetation composition of managed stands (Parendes and Jones., 2000; Watkins et al., 2003; Avon et al., 2010). Roads can act as dispersal conduits for both exotic and native plants (Birdsall et al., 2012; Pauchard and Alaback.,
2004; Tikka et al., 2001), especially if they provide favorable environmental conditions. Significant effects of forest
roads on the roadside are change in abundance of native and alien species (Forman and Alexander, 1998; Gelbard and Belnap, 2003; Hansen and Clevenger, 2005) and some effects on local biodiversity that largely leads to changes in species composition. The high level of disturbance together with specific site conditions such as frequent mowing, soil disturbance, exposure to light, or nutrient-rich and moister soils are favorable to exotic (Parendes, - Jones 2000; Flory and Clay, 2006) and non-forest species (Honnay et al., 2002). On the other hand, moving heavy machineries along forest roads induce disturbance around roads edges. Disturbances caused by change the physical environment and resource availability and rates of organic matter decomposition (Bormann and Likens, 1979), influence on seed production, seed germination, and growth rates of many ground flora species (Reader and Bricker,
1992; Maschinski et al., 1997). Also, It was proved that soil disturbance due to the road construction significantly increased the mineral N content in the first months and/or years after occurrence. This causes the establishment of legumes and other herbaceous communities. Mycorrhizal fungi enhance plant species diversity by increasing the establishment and abundance of different species (Van der Heijden et al., 1998; O’Connor et al., 2002; Parsakhoo et al., 2009). Edge condition shows several impacts in vegetation transmittance. In fact, light availability (Wales, 1972; Ranney et al., 1981) stimulates germination and enhances growth of pioneer or shade-intolerant species (Levenson,
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 544
ISSN 2229-5518
1981) that results in higher productivity, and hence in higher densities and basal areas (Lovejoy et al., 1986; Williams-Linera, 1990).
Far-reaching effects of road influence on plant species diversity which is key issue to integrate the road effect and ecological processes in forest area. According to Laurance et al., (1997) for many phenomena, a reasonable assumption for the maximum penetration of edge effects is 100 m. As Lopez de Casenave et al. (1995) point out, light exposure at the edge stimulates germination and enhances growth of pioneer or light demanding species (Wales, 1972; Levenson, 1981; Ranney et al., 1981; Williams-Linera, 1990b; Williams- Linera, 1993; Aide and - Cavelier, 1994). Previous works have shown that edge environment have increased sapling and tree (Ranney et al.,
1981; Chen et al. 1992), shrub cover (Matlack, 1994), and higher species richness (Brothers and Spingarn, 1992). Tree species distributions may also vary with distance from the forest edge (Whitney and Runkle, 1981; Wales,
1972), or tree mortality may occur from wind throw (Chen et al., 1992; Young and Mitchell, 1994; Laurance et al.,
1998). Most previous studies about edge effects have mainly concentrated on effects of edges on microclimatic and vegetation patterns within the forest community (Williams-Linera, 1990; Euskirchen et al., 2001), and negative effects of edges on forest ecosystems, such as structural damage and alien plant species invasions (Williams-Linera
1990; Ferreira, Laurance, 1997).
Considering previous studies, it is obvious that relatively few ones have focused on the regeneration of light demanding species, especially of dominant species, along an interior-edge gradient. Therefore, the present study aimed at quantifying the spatial distribution of tree regeneration along forest road. We tested the hypothesis that the road as a corridor can change local diversity, and distance from road verge may alter regeneration in down and upslope sides. The aim of this study was to determine the effect of forest roads on diversity of light demanding and shade tolerant species around forest roads.
The study was conducted in Experimental Forest of University of Tehran (Kheyrud Forest) in the middle part of Hyrcanian forest, Northern Iran. Kheyrud forest with an area about 8,000 ha is located in latitude from 36o 27`N to 36o40`N and in longitude from 51o32`E to 51o43`E. The mean annual temperature is 9° C and total annual precipitation of 1380 mm. The forest occupies moderate to steep slopes with deep soil above limestone bedrock. Most stands have an uneven-aged structure where new seedling establishment occurs within canopy gaps (Marvie- Mohadjer, 1976). The silvicultural practice of harvesting is single-tree selection system. Mature forests are dominated by beech (Fagus orientalis with an average volume of 187 m3 ha_1, which represents 74% of total stand volume), Carpinus betulus (21 m3 ha-1, 8%), and Acer velutinum (18 m3ha-1, 7%). Other minor tree species include: Parrotia persica, Acer cappadocicum, Tilia platyphyllos, Ulmus glabra, Cerasus avium, Taxus baccata, and Sorbus torminalis (Marvie-Mohadjer, 2005). The growing season lasts 240 days from April to November. Forest roads, with an average width of 5.5 m and longitudinal gradient ranging from 3 to 8%, are categorized as main forest roads and are used for logging, (Majnounian et al., 2010). The research was carried out on a segment with 16.8 km length.
The segments of road network were selected in a way that forest types in both sides were the same based on the typology map in the scale of 1:25000. After locating sampling points (13 points) with GPS in the field, sampling transects were placed in field from road verge to the forest interior. In each transect six temporary 100 m2 (5×20 m) plots were established at 0, 5, 10, 25, 50 and 100 m distances from the road verge (Fig. 1). In each plot, four 2 × 2 m microplots were set up in the corners for tree regeneration survey. All of transects were in the same management treatment (single tree selection harvesting system). In September 2012, vegetation sampling was conducted in all
520 microplots. Percentage of cover, canopy openness and number of regeneration were recorded in each microplot and in both down-slope, up-slope. We characterized all regenerations into the following size classes: saplings (0-2.5
m height), and thicket (2.5-8 m height) (marvie mohadjer, 2005). The existing species in the study site were categorized into two regeneration categories: shade tolerant species (species that could regenerate in the deep shade found under the closed canopy of these forests), light-demanding species (species that could not regenerate under a closed canopy), as suggested by Swaine and Whitmore (1988), and Moles and Drake (1999).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 545
ISSN 2229-5518
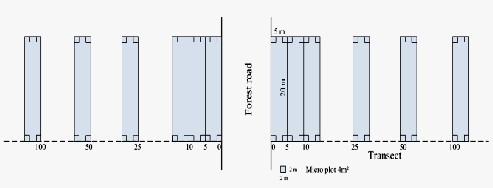
Fig. 1. Vegetation sampling design
First, we explored the data subset for possible outliers. Normality of residuals in each statistical test was
examined by Kolmogrov-Smironov. Significant differences among treatment means were tested using analysis of variances (GLM procedure) and the model was split plot with block randomized design (BRD). SPSS 16 was used to perform statistical analyses. Also, we calculated plant species diversity and species richness indices by PAST software.
Regeneration of eight tree species was identified in microplots. These species consisted of five light-
demanding and three shade-tolerant ones including Acer sp. , Alnus glotinusa, Ulmus glabra, Petrocarya fraxinifolia, Quercus sp. as light demanding species and Fagus orientalis and carpinus betulus as shade tolerant.
ANOVA results showed that distance from the road had significant relationship with density of regeneration While there were no difference between regeneration density of down-slope and up-slope of the road. Also, the species diversity index was not significantly related to down and up slope while these attributes were significant to distance from the road. (P < 0.01)(table.1). Richness index shows no significant difference with any of the factors. Further, Fig 3 showed changes of density of regeneration, diversity and richness indices in six distances from the road. Changes trend of tree regeneration density with distancing from the road showed that, regeneration had the lowest amount in verge of road, but in 25m distance, we observed sharp increase in density of tree regeneration. In other distances regeneration had a similar trend. Accordingly, forest interior had significantly greater number of regeneration than edge plots (Fig.2).
Table 1. Two way ANOVA results for regenerated tree species as dependent factor and distance from the road as fixed factor![]()
![]()
Indices Sources Up/Down Distance![]()
![]()
F P F P Diversity 1.206 ns 0.184 ns
Diversity Simpson 0.596 ns 8.1 ** Shanon-Wiener 0.305 ns 7.515 **![]()
![]()
Richness Menhinick 0.346 ns 0.370 ns Margalef 0.177 ns 1.54 ns
** indicate significance difference at the 5 % probability level, ns – not significant.![]()
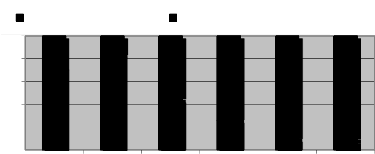
Also trend of species diversity and richness along the forest interior- edge gradients showed that in forest interior, Shannon-Wiener and Simpson indices (Fig.3.a and Fig.3.b) were significantly higher than the edges. Also, diversity has uptrend on first plots and after that, same trend has been observed. Menhinick index had same distribution in all distances while Margalef declined with distance from the road and reached its least value in forest interior (Fig.3.c and Fig.3.d). Distance from road edge was a significant factor on composition of tree regeneration. In road edges, the first plots (0, 5, 10 m distance) had significantly greater number of light demanding species than interior plots.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 546
ISSN 2229-5518
Few light demanding species percolated into forest interiors, whereas shade tolerant species like Fagus orientalis
and Carpinus betulus increased by increasing distance (Fig.4).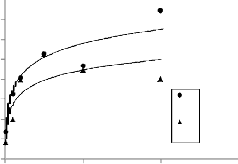
1600
1400
1200
1000
800
600
400
200
0
c ut
fi
ll
0 50 100 150
Distance from road
Fig. 2. Relation of tree regeneration density with distances from the road verge
a)
b)
.80
.70

1.80
.60
1.50
.50
Cut
1.20
.40
.30
Fill
0 20 40 60 80 100 120
Distance from road
.90
.60
.30
Cut
Fill
0 20 40 60 80 100 120
Distance from road
c) d)
3.5
3.3
3.1
2.9
2.7
2.5
2.3
2.1
1.9
1.7
1.5
cut fill

0 1 2 3 4 5 6 7
Distance from road

16
14
12
cut
10
8 fill
6
4
2
0
0 1 2 3 4 5 6 7
Distance from road
Fig. 3. diversity indices, Shanon- Wiener (a), Simpson (b), and richness indices, Menhinick (c), Margalef (d), for tree regenerations at six distances from the edge (0, 5, 10, 25, 50 and 100 m).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 547
ISSN 2229-5518
100%
80%
60%
40%
20%
0%
Shade Tolerate species Light demanding species
0 5 10 25 50 100
Distance from road
Fig. 4. Frequency of Light demanding and Shade tolerant species in different distances from the road
Construction of forest roads creates a tremendous amount of forest edge and gaps, disturbs soil, and decreases the size and density of the forest cover (Sapkota et al., 2009). Formation of forest gaps as a result of forest road
construction is an opportunity to modify system dynamics and ecological processes (Flory, Clay, 2009). According to results, few regenerations were observed in the road verge plots. Lower tree regeneration coverage and species diversity, and more open space were detected in road verge (Fig.2). This can be explained by road construction consequences which may have partly or totally destroyed a population, therefore removing all evidence of previous recruitment events. The frequency and severity of disturbance events may have prevented recruitment (Spooner,
2004). The ability of forest species to establish in the open fields depends on the ability of their seeds to disperse, to germinate, to compete and to survive (Cavallin, Vasseur, 2008). Because of poor soil moisture and nutrients in road shoulder, the dominant plants of this microhabitat are expected to allocate more energy to belowground parts for acquisition of moisture and nutrients (Tilman, 1988). That act as environmental barriers to germination and growth of seeds (Newsome, 1986). Indeed, problems with establishment of regeneration of many species in primary distances from edge appear to be related to problems with competing vegetation and compacted soil. As going
forward within forest, frequency of regeneration may increase. Based on Fig.2, increase in regeneration can be
observed in 25m distance, where the condition is more favorable to establishment of seeds. Our findings agree with previous works (Sheng-Lan Zeng, 2011; Karim, Mallik, 2008; Avon et al., 2010; Avon et al., 2013) and showed distance from road can influence on diversity and richness indices. Dense cover of competing vegetation in the first plots near the road edge, particularly Rubus hyrcanus and Sambucus ebulus and elimination of canopy cover, litter cover, and duff depth, near roadsides has been associated with declining diversity in near roads (Haskell, 2000). In fact, conducive condition near forest edge provides favorable environment for exotic plants, so tree regeneration do not have ability to compete with them and remove them from roadsides. Consequently, according to Fig 3, diversity of regeneration community tend to move to forest interior. In addition, road edges by high soil temperature and solar radiation, low moisture and nutrient content, limiting the establishment and survival of regeneration (Pinard et al.,
1996). Another reason for the absence of regeneration may be grazing and trampling by livestock which exert a negative pressure on some already established species (Barchuk et al., 1998). Although the road construction phase is relatively transitory, the disturbance is destructive with serious consequences, including direct removal of habitats and vegetation (Coffin, 2007). It can be explained by disturbances from roads that change the physicochemical conditions of adjacent environments, and usually increase the availability of nutrients and water and light condition (Forman, Alexander, 1998; Greenberg et al., 1997).
The results showed that forest roads have significant effect on diversity and species composition. So that, light demanding species had lower abundance by increasing distance from road, beside shade tolerant ones have tend to have a presence in the forest. In fact, the distance from road was a significant factor in modeling tree regeneration distribution. Presence of Acer velutinum, Alnus glotinusa and Ulmus glabra along roads showed that light demanding species were more frequent than interior that confirms findings of Delgado et al (2007). As we seen in fig.4, regeneration of light demanding were more in 10 m distance which represents that edge of forest road canopy removal increases duration and intensity of light (Small, McCarthy, 2002) and elevates soil temperatures at the soil surface during the summer (Swank, Vose, 1988). Also, compaction of soil and highest soil bulk density and no
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 548
ISSN 2229-5518
detectable organic matter depth were recorded in the shoulder (Karim, Malik, 2008). Therefore as compared with forest interior, the forest edges have higher air temperature, lower relative humidity and soil moisture, and also encounters wind shear forces (Didham, Lawton, 1999). A combination of the above factors may result in poor tree seedling survival and growth.
It has been widely documented that forest fragmentation resulted from disturbance leads to a greater abundance and richness of light-demanding species near fragmented edges (Laurance, 1991;Williams-Linera et al., 1998; Laurance et al., 2001). When stand canopy closes again, species intolerant to canopy closure only subsist on road verge (Avon et al., 2010). Vegetation associated with forest edges shows several features in response to edge conditions. exposure to light stimulates germination and enhances growth of pioneer or shade-intolerant species (Wales, 1972; Ranney et al., 1981). Scientists know that road effects can reduce the area of interior habitat by changing temperature, moisture, light availability and species composition (Gysel, 1951; Chen et al., 1992; Euskirchen et al., 2001 ). Therefore, the question of the interaction between management practices and road effects on forest diversity is of critical interest for sustainable practices and the conservation of forest communities. Therefore as trees are the most important factor for continuous wood production and sustainability of forest ecosystem, it is necessary to design and construct (i.e. environmentally sound) forest roads carefully and appropriately . It is worthwhile to mention that Hyrcanian forest is the only source of wood production in Iran and sustainability of this forest is of great importance for wood industry.
Marta, Colombia. Restor Ecol 2: 219–229.
Journal of Botany, 25: 269-274.
Transport Geography, 15:396–406.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 549
ISSN 2229-5518
Wisconsin. Forest Ecology and Management, 148:93–108.
13-19.
Mountains. Conservation Biology, 14:57–63.
242.
168.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 550
ISSN 2229-5518
Dynamics in Man-dominated Landscapes.Springer,New York,pp.13-39.
Natural Resources, 34: 77–97.
Maschinski, J., Kolb, T.E., Smith, E., PhilEps, B. (1997). Potential in acts of timber harvesting on a rare understory plant, Clematis hirsutissima var. arizonica. Biological Conservation, 80:49-61.
Ecology, 82: 113–123.
Shorea robusta forests following gap formation, Journal of Forestry Research, 20(1):7-14.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 5, May-2015 551
ISSN 2229-5518
Biogeography Letters, 7:317–333.
Monographs, 42: 451–471.
IJSER © 2015 http://www.ijser.org