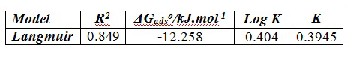International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 683
ISSN 2229-5518
Corrosion Inhibition of Monel in 0.2N HCl
Solution by Tris (hydroxymethyl) aminomethane
Rana Afif Majed Anaee1
Abstract— This study deals with the inhibition of monel in 0.2N HCl solution by Tris (hydroxymethyl) aminomethane by five concentrations
200, 400, 600, 800 and 1000 ppm using electrochemical measurements using Potentiostat. Generally, the results of corrosion test showed that Tris acted as cathodic inhibitor because it was shifted corrosion potentials to more active values. Good efficiencies have been obtained especially in the presence of 600 ppm of Tris. Langmuir adsorption model was achieved, and gave good fitting. Cyclic polarization test indicated the decreasing in hysteresis loop of monel in the presence of Tris especially in the presence of 600 ppm. The small values of equilibrium constant of adsorption process and Gibbs free energy showed the physically adsorption of Tris.
Index Terms—Monel; Tris; Inhibition; Electrochemical methods.
—————————— ——————————
1 INTRODUCTION
ickel–copper alloy is a solid solution binary alloy, com- bining high strength (comparable to structural steel) and toughness over a wide range with excellent resistance to
many corrosive environments. The alloy can be used at tem- peratures up 4278oC and as high as 5388oC in sulfur-free oxi- dizing atmospheres. It has excellent mechanical properties at subzero temperatures. The alloy is readily fabricated and is virtually immune to chloride ion stress corrosion cracking in typical environments. Generally, its corrosion resistance is very good in reducing environments, but poor in oxidizing conditions. The general corrosion resistance of alloy in the nonoxidizing acids, such as sulfuric, hydrochloric, and phos- phoric is improved over that of pure nickel. The influence of oxidizers is the same as for nickel. The alloy is not resistant to oxidizing media such as nitric acid, ferric chloride, chromic acid, wet chlorine, sulfur dioxide, or ammonia. Monel exhibits excellent resistance to hydrofluoric acid solutions at all con- centrations and temperatures. Again, aeration or the presence of oxidizing salts increases the corrosion rate. This alloy is widely used in HF alkylation, is comparatively insensitive to velocity effects, and is widely used for critical parts such as bubble caps or valves that are in contact with flowing acid. Monel is subject to stress corrosion cracking in moist, aerated hydrofluoric or hydrofluorosilicic acid vapor. However, crack- ing is unlikely if the metal is completely immersed in the acid. The corrosion behavior of nickel, Inconel 600 and Inconel 690 was studied in different concentrations of HCl solution and its inhibition by natural rosemary oil using galvanostatic polari- zation techniques. It was found that, HCl accelerate the corro- sion of nickel and its alloys [1]. The inhibition of copper corro- sion by Benzotriazole (BTA) in 5% HCl has been investigated by weight loss technique at different temperatures. Maximum value of surface converge was 0.998 for BTA at 35oC and 15 g/l inhibitor concentration, while the lower value was 0.868 at
55 oC and 1 g/l inhibitor concentration [2].
————————————————
• 1Rana Afif Majed Anaee: Assistant Prof. Dr. at Materials Eng. Dep.- University of Technology/ Iraq-Baghdad, E-mail: dr.rana_afif@yahoo.com , dr.rana.a.anaee@uotechnology.edu.iq
The corrosion inhibition of copper-nickel alloy by Ethylenedi- amine (EDA) and Diethylenetriamine (DETA) in 1.5M HCl has been investigated by weight loss technique at different tem- peratures. Maximum value of inhibitor efficiency was 75% at
35 oC and 0.2 M inhibitor concentration EDA, while the lower value was 4% at 35 oC and 0.01 M inhibitor concentration DE- TA [3].
The corrosion of copper – nickel alloy in hydrochloric acid was investigated at different temperatures, inhibitor concentra- tions and corrosive solution velocities. Weight loss technique was used to evaluate the corrosion rate data [4]. Some indole derivatives are investigated as corrosion inhibitors for nickel in 0.5 M HCl solution using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. A significant decrease in the corrosion rate of nickel was ob- served in the presence of investigated indole derivatives [5]. The corrosion behavior of the nickel electrode was investigat- ed using open circuit potential measurements, galvanostatic, and potentiostatic polarization techniques. The effect of open circuit potential, current densities, NaOH concentration, Cl- anions, and some natural oils e.g. sesame oil, water cress oil, wheat germ oil and almond oil as an inhibitors for corrosion of the nickel in 1x10-2 M NaOH solution was studied [6].
The aim of present work is attempt to inhibit corrosion of Mo- nel in 0.2N HCl solution at room temperature using Tris. Tris is an organic compound with the formula (HOCH2 )3 CNH2 . Tris is extensively used in biochemistry and molecular biolo- gy. In biochemistry, Tris is widely used as a component of buffer solutions, such as in TAE and TBE buffer, especially for solutions of nucleic acids. It contains a primary amine and thus undergoes the reactions associated with typical amines, e.g. condensations with aldehydes.
2 MATERIALS AND METHODS
Ni- alloy was cut into square shape with (1 cm2) area, and made into electrode by pressing a copper wire into a hole on one side and then insulating all but one side with an epoxy resin. The exposed area was grinding on emery papers 500,
800, and 1000 mesh grit. The electrochemical cell was of the usual type with provision for working electrode (Monel), aux-
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 684
ISSN 2229-5518
iliary electrode (Pt electrode), and a Luggin capillary for con-
nection with an SCE reference electrode.
The basic solution was 0.2N HCl solution, HCl obtained by
GCC with purity 35.4% and density 1.19g.cm-3, which prepare
in distilled water.
Electrochemical measurements were performed with a poten-
tiostat WINKING M Lab200 at scan rate 5mV/sec. The main results obtained were expressed in terms of the corrosion po- tentials (Ecorr ) and corrosion current density (icorr ) in addition to calculate the cathodic and anodic Tafel slopes by using ex-
trapolation method.
3 RESULTS AND DISCUSSION
3.1 Electrochemical Behavior
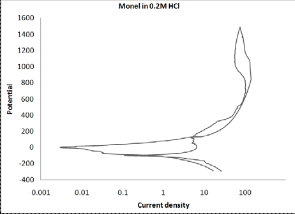
Figure (1) shows the polarization curves of monel in 0.2N HCl solution in the absence and presence of Tris, these curves show the cathodic and anodic regions. At cathodic sites, evolution of hydrogen molecules takes place as follows:
2H+ + 2e →H2 ↑ (1)
While at anodic sites, dissolution of nickel occurs according to the
following reaction:
Ni → Ni2+ + 2e (2)
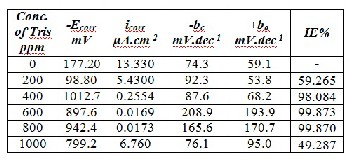
From Tafel plots can be seen that the presence of 600 ppm Tris
shifts the anodic curve to less current density values. Corrosion
parameters of monel in corrosive medium are listed in Table (1).
These data show that the presence of Tris shifts corrosion poten-
tial, in general, toward active direction except in the presence of
200 ppm. All concentrations of Tris shifted the corrosion current
density to less value. Cathodic Tafel slopes were increased; also
anodic Tafel slopes were increased except for 200 ppm of Tris.
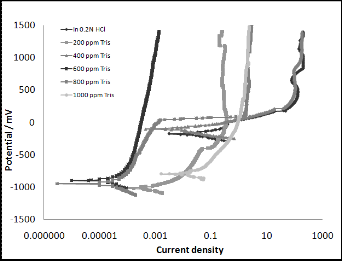
Figure (1) Polarization curves of monel in 0.2N HCl solution in the absence and presence of five concentrations of Tris.
Table (1): Corrosion parameters of Monel in 0.2N HCl solution in absence and presence five concentrations of Tris as inhibi- tor.
From polarization curves can be concluded that Tris acted as ca- thodic inhibitor.
The inhibition efficiency IE (%) can be calculated using the equa- tion given below [7]:
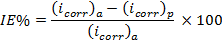 (3)
(3)
Where (icorr)a and (icorr)p are the corrosion current density
(μA.cm-2) in the absence and the presence of the inhibitor, respec-
tively. The data of IE% showed that 600 ppm of Tris had the
highest efficiency. Tris has one amine group and three hydroxyl
groups, these functionality groups have the ability to adsorb on
monel surface.
The active (—:NH2) group contains a pair of unshared electrons
which it donates to the metal surface. The polar amine group dis- places water molecules from the surface. On adsorption, most of the metal surface is covered by the adsorbed water molecules. The inhibitors react by replacing water molecules by organic in-
hibitor molecules as follow [8]:
Org.molecule(aq)+nH2 O(ads)→Org.molecule(ads) +nH2O(soln) (4) Here n represents the number of molecules which are replaced to
accommodate the organic molecule.
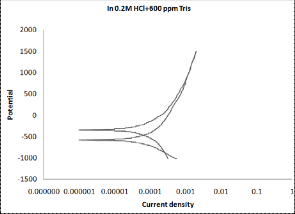
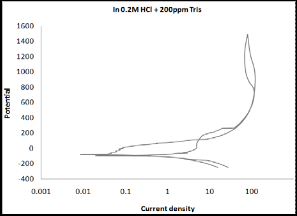
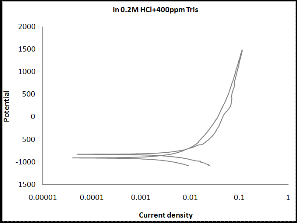
3.2 Cyclic Polarization
Cyclic polarization test was performed to estimate the resistance to pitting corrosion, where the hysteresis loop increases as the susceptibility of material to corrosion increases. Cyclic polariza- tion curves for monel in 0.2N HCl solution in the absence and presence of Tris indicate that the hysteresis loop became smaller in the presence of inhibitors. This result confirms the inhibitive action for Tris. The best curve can be observed in the presence of
600ppm as shown in Figure (2).
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 685
ISSN 2229-5518
(a)
(b)
(c)
(d)
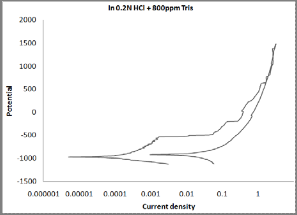
(e)
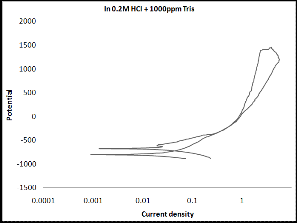
(f)
Figure (2 a-f): Cyclic polarization of monel in 0.2N HCl solu-
tion in the absence and presence of Tris with
five concentrations.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 686
ISSN 2229-5518
3.3 Adsorption Isotherm and Thermodynamic
Calculations
In order to get more information about the mode of adsorp- tion of the respective Tris on monel electrode surface, the data obtained from electrochemical technique have been tested with Langmuir isotherm. The testes indicated that the adsorp- tion of the studied Tris on monel surface is best described by Langmuir. Langmuir adsorption isotherm relationship is rep- resented by equation [9]:
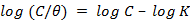
(5)
where C is the concentration of the inhibitor in the bulk elec- trolyte, θ, is the surface coverage by inhibitor molecules and K is the equilibrium constant of adsorption. Plot of log (C/θ) vs. log C is shown in Fig. (3). Values of adsorption parameters deduced from the isotherms are presented in Table (2). From the results obtained, the R2 values for the plot is closer to unity (0.849), indicating that the adsorption of the studied Tris is consistent with the Langmuir adsorption model.
The Langmuir isotherm is based on the assumption that each site of metal surface holds one adsorbed species. Therefore, one adsorbed H2 O molecule is replaced by one molecule of the inhibitor adsorbate on the monel surface.
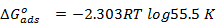
The equilibrium constant of adsorption deduced from the Langmuir adsorption model is related to free energy of ad- sorption (ΔGo ads ) of the inhibitor as follows [9]:
(6)
The value of ΔGoads is shown in Table (2).
The negative values of ΔGoads indicated the spontaneous ad- sorption. Therefore, the adsorption of the studied Tris on mo- nel surface is spontaneous and is consistent with the mecha- nism of electrostatic transfer of charge from the charged inhib- itor’s molecules to charged metal surface which supports
physicsorption.
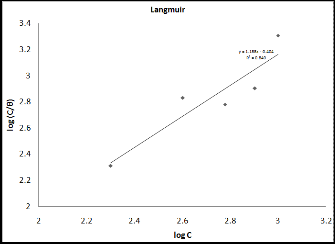
Figure (3): Adsorption isotherm of Tris on monel surface in 0.2N HCl solution.
Table (2): Adsorption parameters for Tris on monel surface.
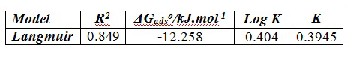
4 CONCLUSION
Tris (hydroxymethyl) aminomethane was used to inhibit the corrosion of Monel in 0.2N HCl solution with five concentra- tions include 200, 400, 600, 800 and 1000 ppm, and gave good efficiencies by electrochemical measurements involve polari- zation test and cyclic polarization. Tris was obeying Langmuir isotherm with small values of equilibrium constant and the change in free energy of adsorption, i.e., the adsorption of Tris is physically adsorption.
REFERENCES
[1] M. Abdallah, S.O. Al Karanee, and A.A. Abdel Fattah, “Corrosion behavior of nickel and its alloys in HCl and its inhibition by natural rosemary oil”, ZAŠTITA MATERIJALA, Vol.50 (2009), broj 4. p.205-
212.
[2] A. Anees Khadom, S. Aprael Yaro, and H. Abdul Amir, “Adsorption
mechanism of benzotriazole for corrosion inhibition of copper-nickel alloy in hydrochloric acid”, J. Chil. Chem. Soc., Vol.55, No.1 (2010), p.150-152.
[3] A. Anees Khadom, A. S. Yaro, A. Y. Musa, A. Mohamad, and A. H.
Kadhum, “Corrosion Inhibition of Copper-nickel Alloy: Experi-
mental and Theoretical Studies”, Journal of the Korean Chemical So- ciety, Vol. 56, No. 4 (2012).
[4] A. A. Khadom, “Effect of Corrosive Solution Motion on Copper – Nickel Alloy Pipe in Presence of Naphthylamine as a Corrosion In- hibitor”, J. Mater. Environ. Sci., Vol.4, No. 4 (2013), p.510-519.
[5] A.S.Fouda, H.Tawfik, N.M.Abdallah and A.M.Ahmd, “Corrosion
Inhibition of Nickel in HCl Solution by Some Indole Derivatives”,
Int. J. Electrochem. Sci., Vol.8 (2013) p. 3390-3405.
[6] M. Abdallah, I. A. Zaafarany, S. Abd El Wanees, and R. Assi, “Corro-
sion Behavior of Nickel Electrode in NaOH Solution and Its Inhibi- tion by Some Natural Oils”, Int. J. Electrochem. Sci., Vol.9 (2014), p.
1071-1086.
[7] K.F. Khaled, and E. Ebenso, “Cerium salt as green corrosion inhibitor for steel in acid medium”, Research on Chemical Intermediates, DOI:
10.1007s 11164-013-1167-3, April 2013.
[8] Zaki Ahmad, “ Principle of corrosion engineering and corrosion
control”, ISBN: 0750659246, Pub. Date: September 2006, Publisher: Elsevier Science & Technology Books.
[9] Da-Quan Zhang, Qi-Rui Cai, Xian-Ming He, Li- Xin Gao and Guo-
Ding Zhou, “Inhibition effect of some amino acids on copper corro- sion in HCl solution”, Materials Chemistry and Physics, Vol.112 (2008), p.353-358.
IJSER © 2015 http://www.ijser.org