International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 467
ISSN 2229-5518
Corrosion Behaviour Of Zinc Plated 1018
SteeI in Tomato Fluid
1Oloruntoba.D.T, 2*Oluwole, O.O, and 1Awheme.O
1Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, Nigeria
2Mechanical Engineering Department,
University of Ibadan, Ibadan, Nigeria.
Abstract-This research work investigated the corrosion resistance of zinc plated 1018 steel in tomato fluid. It simulated the effect of continuous use of the material in a tomato environment where corrosion products are left in place. 1018 steel samples were zinc electroplated at voltages between 0.5V to
0.9V for 5 to 20minutes.The plated samples were then subjected to tomato fluid environment for 30 days. The electrode potentials mV (SCE) were measured every day. Weight loss was determined at intervals of 5 days for the duration of the exposure period. The result showed corrosion attack on the zinc- plated steel, the severity increasing with the increasing weight of zinc coating on substrate. The result showed that thinly plated 1018 steel generally did not have any advantage over unplated steel and were quickly stripped of their zinc plating with resultant corrosion of the underlying steel substrate. Heavily zinc-plating steel was observed to offer some form of protection for the plated steel. The pH of the tomato solution which initially was acidic was observed to progress to neutrality after 5 days and then became slightly alkaline at the end of the thirty days test (because of corrosion product contamination of the tomato) contributing to the reduced corrosion rates in the plated samples after 10 day. Zinc coating of 1018 steel was found to be unsuitable for the fabrication of tomato processing machinery in this environment.
Index terms: corrosion resistance, zinc plating, low carbon steel, Tomato fluid
*Corresponding author: Dr.O.Oluwole.
e-mail: oluwoleo2@asme.org. Tel: +234(0)8033899701
1 INTRODUCTION
Corrosion has been established in uncoated mild steel used in machinery for agro-processing[1],[2]. Previous work on zinc plating on steel used for cocoa and cassava processing machinery showed that zinc did not offer much protection because of the presence of ethanoic acid and cyanide in the respective fluids during processing [3],[4]. Tomatoes are the fruits of the plant lycopersicon esculentum and are one of the most widely grown of the tropical vegetables. The predominant acids are citric and malic glutanic acid. Methionine and S-methylmethionine are also present [5]. The presence of these acids is likely to cause corrosion of
the machinery used in the processing and storage of the
tomato juice. The average analysis of tomatoes fluid is as shown in table 2. The objective of the research is to investigate the corrosion of zinc plated low carbon steel in tomato fluid.
2 MATERIALS AND METHOD
2.1 Material
The material used in this investigation is a low carbon steel substrate of length 1metre and 20 millimeter diameter. It was obtained from Nigeria Machine Tools Limited, Oshogbo, with batch number “DL 150”, part number “01-
160-61”. The chemical composition of the steel is shown in
Table 1.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 468
ISSN 2229-5518
Table1: Chemical Composition (wt%) of low Carbon Steel
Substrate
Table 2: Tomato Fluid Properties (Average)
Alloy Elements | Composition(wt%) |
Carbon | 0.180 |
Silicon | 0.100 |
Sulphur | 0.031 |
Phosphorus | 0.038 |
Copper | 0.230 |
Chromium | 0.100 |
Tin | 0.008 |
Vanadium | 0.006 |
Iron | 99.31 |
2.2 Method
2.2.1 Preparation Of Specimens (Surface)
The 1018 steel was used in as received condition. The samples were machined into cylindrical pieces of 20mm diameter and 20mm long. The sample surface were treated by abrading them through successive grades of silicon carbide papers of grades 60,120,320,400 and 600grit, and finally on the cloth grade. They were rinsed in distilled water and then in acetone before drying. The prepared sample were then stored in desiccators until they were needed for the experiments.
2.2.2 Preparation of the tomato fluid
The environment under study is tomato fluid. Fresh tomatoes were procured, and the fluid was manually squeezed out into a clean bowl and stored in a 10 litre
gallon and labeled. The chemical composition of each of the tomato fluid was carried out at the Crop, Soil and Pest’s departmental laboratory of the Federal University of Technology, Akure (FUTA). The result is shown in Table 2.
2.2.3 Samples Pre-Treatment before Electroplating
Operations
The samples were removed from the desiccators in turn and pickled in 0.5M H2 SO4 for 2 minutes, then rinsed in distilled water before degreasing in an 100 litre electrolytic degreasing tank containing 200g KOH and 100g NaOH in distilled water for 2 minutes, after which the samples were rinsed in distilled water. The samples were weighed using a digital weighing balance model Scout Pro SPU402 of accuracy ± 0 .01g and the weight was recorded as the initial weight.
2.2.4 Electroplating Operation
The laboratory zinc electroplating bath was stirred with the aid of a stirrer for 1 minute. The sample already attached to the flexible copper wire was then hanged on the cathode arm of the zinc electroplating bath after which the electroplating rectifier was switched on. The electroplating rectifier was regulated to obtain 0.5V. The steel sample was allowed to stay in the plating bath for 5 minutes and then
removed, dried in air and reweighed. This procedure was
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 469
ISSN 2229-5518
repeated for 10, 15 and 20 minutes of electroplating time. This procedure was repeated using voltages of 0.6, 0.7, 0.8 and 0.9V and varying electroplating times for 5, 10, 15 and
20 minutes. This process resulted in a series of zinc coatings of different coating weights on the steel.
2.2.5 Corrosion Resistance in tomato fluids
The corrosion environment for examining the corrosion protection performance of the zinc electroplated samples was tomato fluid. The zinc electroplated samples were immersed in tomato fluid for durations up to 30 days, including an un-plated sample as control. Electrode potential (mV) measurements between the sample surface and the corrosive environment were done at regular interval of 24 hours using a DT8300D digital multimeter with a zinc electrode used as a reference electrode. The reference electrode was not left in the cell for the duration of the experiment but used only at time of measurement of potential then afterwards removed. Values obtained were converted to Saturated Calomel Electrode (SCE) values[6]. Weight change determination was used in the calculation of
corrosion rate due to its simplicity [7],[1],[3],[4]. The
corrosion samples were removed from the corrosion environment (tomato fluid) with the aid of a tong after which the samples were properly cleaned in distilled water and then dried with a cotton wool. The dried samples were weighed with a digital chemical weighing balance and recorded and this continued at regular intervals of five days.
3.0 RESULTS AND DISCUSSION
3.1 Results
The electroplating mass gains, and corresponding coating thicknesses value, for the zinc plated steel sample at various voltages and times are shown in Table 3 and in Figure 1. It can be seen that the resulting zinc coating thickness varied effectively linearly with coating time and increased with coating voltage. The coating thickness varied from about 1 µm to 30 µm. Table 4 shows the variation in pH values for tomato fluid during the corrosion test period. It shows the pH of the corrosive fluid changing from acidic to neutrality and then to alkalinity at the end of the corrosion test.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 470
ISSN 2229-5518
Table 3: ELECTROPLATING WEIGHT (g) VALUES OF ZINC PLATED LOW CARBON STEEL SAMPLE AT VARIOUS VOLTAGE AND TIME
Sample Number | Voltage (V) | Time (min.) | Weight gain (g) | Coating mass per unit area (mg/cm2) | Coating thickness (µm) |
1 | 0.50 | 5.0 | 0.01 | 0.6 | 0.8 |
2 | 0.50 | 10.0 | 0.03 | 1.7 | 2.3 |
3 | 0.50 | 15.0 | 0.06 | 3.3 | 4.7 |
4 | 0.50 | 20.0 | 0.17 | 9.4 | 13.2 |
5 | 0.60 | 5.0 | 0.03 | 1.6 | 2.3 |
6 | 0.60 | 10.0 | 0.08 | 4.3 | 6.1 |
7 | 0.60 | 15.0 | 0.10 | 5.6 | 7.9 |
8 | 0.60 | 20.0 | 0.22 | 12.2 | 17.1 |
9 | 0.70 | 5.0 | 0.04 | 2.2 | 3.1 |
10 | 0.70 | 10.0 | 0.11 | 6.0 | 8.4 |
11 | 0.70 | 15.0 | 0.13 | 7.3 | 10.2 |
12 | 0.70 | 20.0 | 0.35 | 18.9 | 26.5 |
13 | 0.80 | 5.0 | 0.06 | 3.5 | 4.8 |
14 | 0.80 | 10.0 | 0.13 | 7.0 | 9.9 |
15 | 0.80 | 15.0 | 0.23 | 13.0 | 18.2 |
16 | 0.80 | 20.0 | 0.39 | 21.9 | 30.6 |
17 | 0.90 | 5.0 | 0.08 | 4.4 | 6.1 |
18 | 0.90 | 10.0 | 0.19 | 11.0 | 15.4 |
19 | 0.90 | 15.0 | 0.33 | 17.6 | 24.6 |
Table 4: Variation In pH Values For Tomato Fluid During The Test Period.
Exposure time (days) | Change in pH |
0 | - |
5 | 3.14 |
10 | 4.12 |
15 | 4.78 |
20 | 5.92 |
25 | 6.38 |
30 | 6.98 |
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 471
ISSN 2229-5518
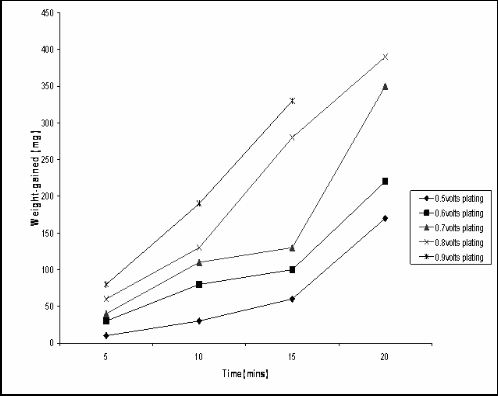
Fig.1: Plot of weight gained against time for zinc-plated low carbon steel samples at various voltages and times
Figure 2 shows the variation in the electrode potential in
mV obtained for un-plated and zinc plated low carbon steel samples at various voltages and electroplating times. For most samples, except those with the thinnest coatings, the electrode potential starts at values consistent with that of zinc and relatively rapidly moves to values consistent with
the exposure of steel, although not necessarily over the
entire metal surface. An unusual potential excursion
occurred for all samples between about 7 and 10 days of exposure but there is no explanation for this and it must be assumed to be experimental error. Generally, the measurement of potential with time under these conditions resulted in data that appeared to be indistinguishable
experimentally from each other.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 472
ISSN 2229-5518
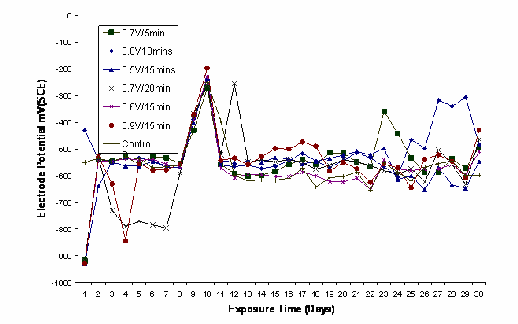
Fig.2: Plot of Electrode Potential against Exposure Time in Tomato Fluid for unplated and zinc–plated low carbon steel samples electroplated at various voltages and times.
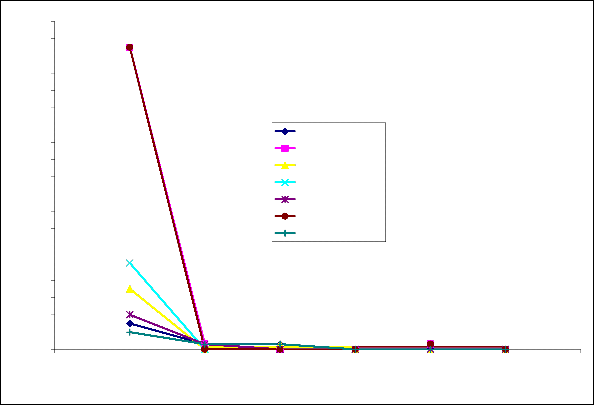
Figure 3 shows the variation of corrosion rate in mm/yr for
the various zinc plated low carbon steel samples as well as the corrosion rate of the un-plated sample immersed in tomato fluid (control sample). It shows a relatively high corrosion rate experienced by the zinc plated steel in the over the first 5 days of immersion. Interestingly, compared with the corrosion of bare steel, the corrosion of zinc was much higher (up to 7 times greater) with greater corrosion
observed as the thickness of the zinc coating increased.
Figure 4 tabulates the increase in weight caused by initial
zinc plating compared with the loss of weight caused by subsequent corrosion. It is quite evident that thin coatings of zinc on steel are not beneficial and do not protect the steel as they are quickly stripped off. Thicker coatings were found suitable as they were not stripped off during the duration of the test. However, the corrosion rate of the zinc coating itself is rather high.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 473
ISSN 2229-5518
1.9
1.8
1.7
1.6
1.5
1.4
1.3
1.2
1.1
1
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
0.7V/5 min
0.7V/20 min
0.8V/10 min
0.5V/15 min
0.6V/15 min
0.9V/15 min
Unplated steel
0 5 10 15 20 25 30 35
Exposure Time (Days)
Fig.3: Plot of corrosion rate against exposure time in tomato fluid for unplated and zinc plated
low carbon steels electroplated at various voltages and time.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 474
ISSN 2229-5518
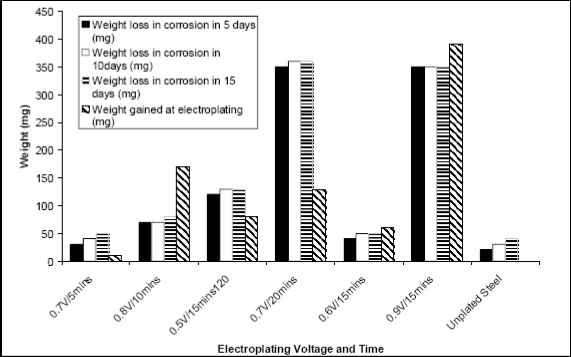
Fig.4: Plot showing effect of weight of zinc-plating on corrosion resistance of zinc plated low carbon steel in Tomato Fluid
3.2 Discussion
3.2.1 Effect of Exposure Time on Potential
With the exception of the un-plated sample, which showed a fairly constant potential characteristic of bare steel (-
550mV, SCE) from initial immersion, the samples with the lowest coating thicknesses (0.5V/15mins, 0.6V/15mins,
0.7V/5mins and 0.8/10mins; i.e. generally less than about 10
µm) showed potential moving within 1-2 days into the potential region characteristic of steel. Note that although the pH was changing during the immersion, the extent of the change is deemed to be too small to be significant. An increase in coating weight only marginally affected the variation in potential with time with the rise in potential to that characteristic of steel being delayed for 5-6 days. Eventually, the open circuit potentials of all coating weights lay in the range for that of steel illustrating that exposure of
the substrate had occurred. Visible corrosion products for
steel (i.e. rust) were apparent from early immersion for the majority of the samples.
3.2.2 Effect of Exposure Time on Corrosion Rate
The extent of susceptibility to corrosion in natural fluids depends on the aggressiveness of chemical reactivities, transport properties of environment, concentration of corrosion species in the medium (pH), the metallurgy of the alloy sample and temperature of the corrosion medium[8]. In the tomato fluid, the corrosion rates of all the samples decreased with time tending to relatively low rate after 10 days of exposure, Figure 3. This can be attributed to the formation of a layer of corrosion product on all the sample surfaces [9],[10], from the corrosion products and the changing of the pH of the corrosive environment to basicity.
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 475
ISSN 2229-5518
3.2.3 Suitability of Zinc coatings in a Tomato
Environment
It is evident that uncoated steel is suitable for a material of construction for tomato processing equipment with a corrosion rate not exceeding 0.1mm/yr [11],[12].This work tested the use of zinc coatings as a potential protective coating. Thinly coated zinc corroded rapidly in this environment and certainly makes low coating thicknesses ineffective. Thicker coating proved effective though for the time of test. The reason for the relatively rapid corrosion of zinc is undoubtedly due to the easy dissolution of zinc in the citric and malic glutanic acids and consequently more rapid corrosion. Unfortunately, results in zinc being an unsuitable material for the protection of steel in tomato fluid.
4.0 CONCLUSION
Uncoated 1018 steel has been found to be suitable for use in tomato food processing due to its relatively low corrosion rate(0.1mm/yr).
Effect of using galvanized steel as constructional material for tomato processing was studied.
Unfortunately, due to the citric and malic glutanic acids content of tomato, zinc had an unacceptably high corrosion rate that was greater than the underlying steel. Thinly coated steels were soon depleted of the
zinc coatings but thicker plated 1018 steel was protective
in the tomato environment.
REFERENCES
[1] Adebiyi, K.A, Hammed, K.A, and Ajayi, E. O. (2003). “Predictive model for evaluating corrosion rate of mild steel in six environments”, LAUTECH Journal of Engineering andTechnology. In Jekayinfa, S.O.(Eds),Effect of cassava fluid on corrosion performance of mild steel,Vol
52, No.5,pp.286-292.
[2]Jekayinfa S.O., Waheed M.A., Adebiyi K.A. and Adebiyi F.T. (2005). “Effect of cassava fluid on corrosion performance of mild steel”. Anti- Corrosion Methods andMaterials, Volume 52, No. 5,pp.286-292.
[3] Oluwole,O.O., Oloruntoba,D.T and Awheme.O(2008)"Effect of zinc plating of low carbon steel on corrosion resistance in cocoa fluid environment" Materials and Design, Elsevier,29(6),1266-1274. [4]Oluwole,O.O, Oloruntoba.D.T and Awheme.O(2008)"Effect of zinc plating of low carbon steel on corrosion resistance in cassava fluid environment” Corrosion Engineering Science and Technology, Institute of Materials, Minerals and Mining, UK. 43(4),320-323.
[5]Ihekoronye A.I and Ngoddy P.O (1985). “Integrated food science
and technology for the tropics”. Macmillan, London, UK. [6]Hibbert D.B. and JamesA.M.(1984). “ Dictionary of Electrochemistry”. Macmillan Press, London.
[7]Sodile J.L. (2002). “The effect of surface finish on the corrosion rate of steel”.Nigerian Journal of Engineering Research and Development, Vol.1 No.2, pp.1-9.
[8]Loto C.A. and Adesomo M.A. (1988) “ The corrosion of mild steel in maize juice, acidified maize juice and acetic acid environment”. African Journal of Science and Technology Series A, Vol.7 No.1.
[9]Fontana M.G. and Green N.D. (1974) “ Corrosion Engineering,”. McGraw-Hill, International Book Company, U.K.
[10]Scully J.C. (1975) “The fundamental of corrosion”, PergamoN Press, Oxford.
[11]Moore.J.J (1981) “Chemical Metallurgy” Butterworths,London.p.300-301.
[12]Singh.V(1999) “Physical Metallurgy” Standard Publishers, New
Delhi.p.719-720
IJSER © 2014 http://www.ijser.org



