International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2852
ISSN 2229-5518
Computer Simulation Applied to
Separate Ethanol from Ethyl Acetate by Normal Distillation Process
aTaha Mahdi*
Abstract- Distillation is one of the oldest and most important separation processes that it is used in the chemical and petrochemical industries. The objective of this work is to separate a highly non-ideal mixture of ethanol from ethyl acetate by continuous normal distillation column using computational simulation, Aspen Plus software as a simulator. The effects of the number of stages, location of the feed stage and reflux ratio on the purity of products were studied. Calculation of the vapor-liquid equilibrium and relative volatility for this system was done by the NRTL model and the data of VLE compared with literatures. The results indicated that the mole fraction of ethyl acetate in the distillate is less than or equal to 0.555. The separation of this mixture by normal distillation column is to provide evidence of the poor capability of the ordinary distillation process in the direct separation of the both components.
Keywords- Simulation, aspen plus, distillation process, non-ideal mixture, VLE
—————————— ——————————
1 INTRODUCTION…………………………

The separation of liquid mixtures is an exhaustively investigated old area of the different engineering sciences. Distillation technique is the most widely used in industrial separation liquid mixture and is at the heart of the separation processes in many chemical and petroleum plants such as extraction, absorption, membrane base, crystallization technologies and so on. Gmehling [1] pointed out that 90% of binary and multi-component mixtures in industries were separated by using distillation technique. The advantages of distillation process are, many products throughput with high purity, flexibility to design requirement and ability to operate with any feed concentration depends on differences in volatility between the components [2]. The liquid is brought to boiling and great relative volatility is easily separated and condensed to form product.
————————————————
• Faculty of chemical engineering, Universiti
Teknologi Malaysia, Skudai 81310, Malaysia
• Corresponding author. *Taha Mahdi
Abdulhamza, tahamahdi91@yahoo.com
Although distillation is the most widely used separation technique in chemical and petroleum industries, not all liquid mixtures can be separated, especially azeotropic and close boiling point mixtures. For separation of azeotropic systems with simple distillation process is either too expensive or impossible because of a high reflux or large column requirements [3]. There are various methods for separating non ideal mixtures. Distillation methods are the oldest and most used, either by pressure variation such as pressure swing distillation process or the addition of third component as separating agent such as azeotropic distillation and extraction distillation processes, that may change the phase equilibrium of the mixture. Other alternative separation techniques for azeotropic mixtures are membrane separation and usually combined prevaporation process. Hybrid separation processes can also be used if distillation is combined with other separation processes.
Ethyl acetate is one of the most popular solvents and widely used in the manufacture of nitrocellulose lacquers, varnishes and thinners. Ethyl acetate is an important component in extractants for the concentration
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2853
ISSN 2229-5518
and purification of antibiotics [4]. It is also used as an intermediate in the manufacture of various drugs. It finds extensive use in the preparation of synthetic fruit essences, flavors and perfumes. Furthermore, Ethyl acetate is used in the manufacture of adhesives, cleaning fluids, inks, nail-polish removers and silk, coated papers, explosives, artificial leather, photographic films and plates [5], [6].
Manufacturing of ethyl acetate in industries is done by using several routes such as by alkylation of acetic acid with ethylene in the presence of sulfuric acid as a catalyst, dehydrogenation of ethanol, 2C2H5OH → CH3COOC2H5 + 2H2, oxidation of ethanol
2C2H5OH+O2→CH3COOC2H5+2H2O in the presence of Graphite nanofibers (GNFs) catalyst, reaction of ethanol with acetaldehyed
2C2H5OH → CH3COOC2H5 in the presence of an alkoxide catalyst and in fact, ethyl acetate is usually on the large scale made from the
esterification of ethanol with acetic acid
(CH3C(O)OH+CH3CH2OH→CH3C(O)OCH2
CH3+H2O) [7]. Since these methods used
ethanol to produce ethyl acetate and the
reactant components do not totally convert to
ester, especially the latest reaction in about
65% yield, the residual of unconverted ethanol
form azeotrope with the product of ethyl acetate [8].
Separation of ethanol (ETOH) from ethyl acetate (ETAC) mixture is among the most important and most difficult separation processes in petrochemical industry. The boiling points of ETAC and ETOH mixture are
78.650C and 77.35 0C respectively (which
mean that the difference is only 1.3 0C). The
separation of this mixture by conventional
distillation process is impossible due to their close boiling points and formation of an azeotropic mixture (45 mole % ethanol).
In recent years, the use of different separation techniques to separate ethanol (ETOH) and ethyl acetate (ETAC) mixture have attracted the attention of researchers to develop new processes because of the increasing demands in various industries and high purity of ETAC can only be obtained under a complex process control. There have been several studies and articles in various journals and chapters in books discussing on the separation of ETAC and ETOH mixture.
These purification techniques of ETAC have been investigated, such as azeotropic distillation [9], [10], extractive distillation [11], pressure swing distillation [12], membrane separation (pervaporation) [13], [14] and the latest technique by ionic liquids [15]. However, until now, there is no published deal about the separation of ETAC and ETOH mixture by normal distillation column.
The purpose of this article is to separate ETAC and ETOH mixture by normal continual distillation column, whereby studied by simulation on ASPEN PLUS software. Moreover, this article represents the basic and fundamental studies to help the researchers to develop their technologies that deal with the separation of ETAC and ETOH mixture and in order to compare its results with this study. Furthermore, this work provides evidence for the poor capability of the ordinary distillation process in the direct separation of this mixture. Sensitivity analyses are performed to study the effects of various operating parameters on the purity of ETAC. The Sensitivity analyses include a number of stages, location of the feed stage and reflux ratio that will assist to obtain the desired results. The thermodynamic package using NRTL model is discussed to evaluate the vapor-liquid equilibrium (VLE) and relative volatility.
Process simulation technology in chemical engineering is one of the effective tools to predict the behavior of a process by using basic engineering relationships, such as mass and energy balances, and chemical equilibrium. Process simulation is helpful throughout the entire life of a process, from research and development through process design to production. Efficient design of distillation equipment requires quantitative understanding of vapor liquid equilibria. With simulation one can design better plants and increase the profitability in the existing plants. Nowadays computational simulators are able to accurately represent the most complex industrial processes by using the commercial Aspen Plus software.
2 PROCESS SIMULATION
Continuous distillation column module from the flow sheet simulator Aspen Plus is
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2854
ISSN 2229-5518
used to simulate the separation of ethanol (ETOH) from ethyl acetate (ETAC). Since the system is highly non-ideal, the RADFRAC is used to encounter slow or difficult convergence for highly non-ideal systems using the standard algorithm [16]. A binary feed mixture of ETOH and ETAC at mole fraction 0.3 and 0.7 respectively, is to be separated in a tray distillation column containing 20 trays including reboiler and condenser. The feed is introduced at a rate 100 kmol/hr with 1 bar pressure and saturated liquid. Total condenser is being used with a reflux ratio of 1. The pressure in each stage, as well as in the condenser and reboiler was fixed at 1 bar. The flowsheet for the simulation distillation process is shown in Figure 1.
Figure 1. Process flow diagram for the distillation column
Table 1: Data results for simulation of distillation column
The detailed simulation results are given in Table 1. During normal operation conditions, for separation of ETAC and ETOH mixture, the results indicate that it is more difficult to separate this mixture. The mole fraction of ETAC in the distillate is less than or equal to 0.555. This separation process for ETAC with low purity is feasible because it’s formed with ETOH, a minimum boiling point azeotrope about (45 mole
% ETOH) and close boiling point (the difference is only 1.3oC).
3 Sensitivity analysis results
For this simulation, it was required to study the effects of changing the operating parameters and observe the resulting change in behavior for the outlet as mol fraction of ETAC in the top. The parameters studies conducted for this distillation simulation are the reflux ratio, number of stages and varying feed stage location.
3.1 Reflux ratio
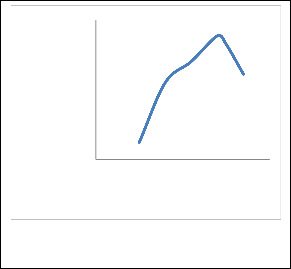
With the operation conditions and other variables held constant, it was required to study how changing the reflux ratio in the column affected the outlet parameters. The reflux ratio varied from 0.5 to 3.5, the mol fraction of ETAC was considered to check for the best result. After the simulation was run, a graph was plotted between mol fraction of ETAC and reflux ratio, which are shown in Figure 2.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2855
ISSN 2229-5518
0.555
0.5545
0.554
0.5535
0.553
0.5525
0 1 2 3 4
Reflux ratio
difference between the highest (0.5538) and lowest (0.5532) values mole fraction of ETAC.
0.5539
0.5538
0.5537
0.5536
0.5535
0.5534
0.5533
0.5532
Figure 2. Reflux ratio influence on the mole
fraction of ETAC.
0 5 10 15 20
location of feed stage
From the above graph, it can be seen that an increase in the reflux ratio gives an overall increase of mole fraction of ETAC in the distillate stream. It was observed that as the value of reflux ratio was increased from 0.5, the mole fraction of ETAC rapidly rose until a reflux ratio of 1.5 was reached, after that it became almost stable until a reflux ratio of 2.5, and then reached the maximum mole fraction of ETAC at 3, after this point it decreased with an increase in reflux ratio. In general, the highest mole fraction of ETAC does not reach more than 0.555 and its lowest is not less than 0.55. That means the reflux ratio is not a great influence on the purity of ETAC.
3.2. Feed stage location
The second parametric study was concerned with examining the effects of having the feed stream enter at differing stages. Feed tray location was varied from 5 to 17 and the graphs were plotted between mol fraction of ETAC and the feed tray location as shown in Figure 3. This graph displays what results, varying the feed location has on the outlet parameter.
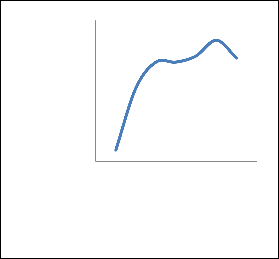
Figure 3. Feed stage location influence on the mole fraction of ETAC.
3.3 Number of stages
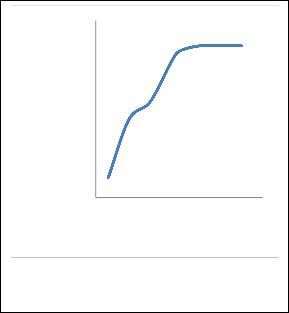
The third study was affiliated with observing the effects on mole fraction of ethyl acetate caused by varying the number of the stages in the column. The number of trays varied from 3 to 35 and graphs between outlet parameter and the number of trays to analyze the results, as shown in Figure 4.
0.5545
0.554
0.5535
0.553
0.5525
0.552
0.5515
0.551
It can be seen that having the feed stream enter the tower at a higher stage number (lower in the tower) will result in less purity of ethyl acetate being brought off the tower in the distillate. As the feed tray location was increased, it was found that the mole fraction of ethyl acetate increased suddenly until the value of 14, after that it steadily decreased until 17. To conclude, this graph shows that there is no great
0 10 20 30 40
number of stages
Figure 4. Number of stages influence on the mole fraction of ETAC.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2856
ISSN 2229-5518
In this process also, the variation of mole fraction of ETAC in distillate was similar. When the number of stages was increased, the mole fraction increased suddenly until the number of stage reached 20, after this stage the change in mole fraction was negligible. It can be observed than an increase in the number of stages leads to a slight decrease in the mole fraction of ETAC (the difference only 0.0026).
4 VAPOR LIQUID EQUILIBRIUM
The Vapor liquid equilibrium (VLE) of the ethanol and ethyl acetate system was determined simultaneously. The NRTL model is selected to describe the VLE of the system. This model is the most appropriate, especially in the case of binary mixtures containing organic components. Figure
5 shows the simulation VLE data for the system
ethyl acetate and ethanol was measured and compared with the experimental data literature [17], [18], [19]. The equilibrium data agree well with those of literature. As seen from this diagram, this system forms the minimum boiling azeotrope, which is about 55% mole of ethyl acetate. This means that the separation of this system in normal distillation column would be difficult or impossible to obtain high purity of ethyl acetate.
Table 2: Vapor–liquid equilibrium data for ethyl acetate (1) +
ethanol (2) system at 15 psia
1
0.8
0.6
0.4
0.2
0
0 0.2 0.4 0.6 x1
0.8 1

together with the relative volatility and activity coefficient of the ethyl acetate (1) and ethanol (2) system obtained are given in Table 2.
The activity coefficient γ for the ethyl acetate (1) and ethanol (2) systems were calculated from NRTL equations:
Figure 5. Binary xy diagram of ethyl acetate +
ethanol systems at 15 psia; (♦ ) this work, (■ )
from [19], (● ) from [18] and (▲) from [17].
lnγ1 = x2 �τ �
lnγ2 = x2 �τ �
G21 2

� +
x1+x2G21
G12 2

� +
x2+x1G12

G12τ12
(x2+x1G12)2

G21τ21
(x1+x2G21)2
� (1)
� (2)

Here,
G12 = exp(−ατ12 ) ; G21 = exp(−ατ21)
And
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2857
ISSN 2229-5518
τ12 =

b12
RT
; τ21 =

b21
RT
understanding that it is impossible to separate this mixture by normal distillation process.
The relative volatility α, for binary systems,
was calculated from the following equation:
o
By implementing the recommendations of this paper, from this work, the researchers in this
yi P = γi Po
K value
xi or

yi =
xi

γi Pi (3)
P
o
field were able to start and quickly execute their novel separation technologies, to separate ethanol and ethyl acetate mixture.
Relative volatility

ki = =
xi

γi Pi
P
(4)
ACKNOWLEDGMENT
The author wish to thank Universiti Teknologi
Malaysia. This work was supported by my
y1�x

α12 = y 1
(5)
father.
Thus
α12 =
2�x2

k1 =
k2
γ1 Po

o (6)
2 2
Reference
1. Gmehling, J., et al., A data bank for azeotropic data—status and
Where yi and xi represent the mole fraction of component i in the vapor and liquid phase
o
applications. Fluid phase equilibria,
1995. 103(1): p. 51-76.
respectively; P is the total pressure; Pi
vapor pressure of pure component ( i ).
is the
2. Perry, R. and D. Green, Chemical
engineers’ handbook7th ed, 1997, New
From above equations the relative volatility of ethyl acetate to ethanol was found only 1.21, therefore the separation of this mixture is impossible by conventional distillation system due to their low relative volatility and azeotropic formations.
5 CONCLUSIONS
By using the process simulation software Aspen plus, a study separation of ethanol from ethyl acetate via normal distillation column is presented. The thermodynamic equations used here are NRTL equations to determine vapor liquid equilibrium and relative volatility between ethanol and ethyl acetate. Sensitivity analysis allowed determining the most operating conditions for the separation of this system to obtain the best conditions and configuration for distillation column. The discussion includes the influences of the parameters including reflux ratio, feed stage location and number of stages upon the performances of the column. However, the most important outcome is that the best result obtained from this work of purity of ethyl acetate was 55.5 mol %. That means, any factor cannot improve the purity of ethyl acetate even under the best operation conditions because this system forms azeotrope point and has close boiling point. The study gives us a clear
York: McGraw-Hill.
3. Richardson, J.F., J.H. Harker, and J.R.
Backhurst, Coulson and Richardson's chemical engineering: Particle technology and separation processes.
2002.
4. Zereshki, S., et al., Pervaporation separation of MeOH/MTBE mixtures with modified PEEK membrane: Effect of operating conditions. Journal of Membrane Science, 2011. 371(1): p. 1-
9.
5. Schierow, L.-J. The Toxic Substances
Control Act (TSCA): Implementation and New Challenges. 2008. Congressional Research Service, Library of Congress.
6. Yonghong, L., L. Jiaqi, and M. Peisheng, Study on the synthesis of pure ethyl acetate by an extractive reactive distillation process. Petrochemical Technology, 1996. 10.
7. Magan, N. and P. Evans, Volatiles as an indicator of fungal activity and differentiation between species, and the potential use of electronic nose
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2858
ISSN 2229-5518
technology for early detection of grain spoilage. Journal of Stored Products Research, 2000. 36(4): p. 319-340.
8. Hassan, A., et al., METHOD OF PRODUCING ETHYL ACETATE, 2009, Google Patents.
9. Berg, L., Separation of ethyl acetate from ethanol by azeotropic distillation,
1999, Google Patents.
10. Harrison, J.M., SEPARATION OF ETHYL ACETATE AND ETHANOL BY, 1953, Google Patents.
11. Nieuwoudt, I. and B. Van Dyk, Separation of ethanol mixtures by extractive distillation, 2002, Google Patents.
12. Colley, S.W., et al., Process, 2009, Google Patents.
13. Sato, K., K. Sugimoto, and T. Nakane, Separation of ethanol/ethyl acetate mixture by pervaporation at 100–130° C through NaY zeolite membrane for industrial purpose. Microporous and Mesoporous Materials, 2008. 115(1): p.
170-175.
14. Zhang, X.H., et al., Pervaporation dehydration of ethyl acetate/ethanol/water azeotrope using chitosan/poly (vinyl pyrrolidone) blend membranes. Journal of Membrane Science, 2009. 327(1): p. 274-280.
15. Zhang, D.L., et al., Separation of ethyl acetate-ethanol azeotropic mixture using hydrophilic ionic liquids. Industrial
& Engineering Chemistry Research,
2008. 47(6): p. 1995-2001.
16. Altman, E., et al., Phase Equilibria for Reactive Distillation of Propyl Propanoate. Pure Component Property Data, Vapor− Liquid Equilibria, and Liquid− Liquid Equilibria. Journal of Chemical & Engineering Data, 2011.
56(5): p. 2322-2328.
17. Kumar, S., Modelling and Simulation of Ethyl Acetate Reactive Distillation Column using ASPEN PLUS, 2010.
18. Tu, C.-H., Y.-S. Wu, and F.-C. Ou, Effect of
1, 2-propanediol on the vapor-liquid
equilibria of the ethyl acetate+ ethanol system at 101.3 kPa. Fluid phase equilibria, 1997. 130(1): p. 243-252.
19. Topphoff, M., J. Kiepe, and J. Gmehling, Effects of lithium nitrate on the vapor- liquid equilibria of methyl acetate+ methanol and ethyl acetate+ ethanol. Journal of Chemical & Engineering Data, 2001. 46(5): p. 1333-1337.
IJSER © 2013 http://www.ijser.org






