International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 1
ISSN 2229-5518
Chitin is the second most abundant natural polymer next to cellulose. The main sources exploited are two marine crustaceans, shrimp and crabs. Chitosan is a natural based polymer, obtained by alkaline deacetylation of chitin, which presents excellent biological properties such as biodegradability and immunological, antibacterial and wound-healing activity. Chitosan is soluble in acidic aquiousmedia and issued for many applications. This review emphasizes recent papers various derivatives of chitosan and their value added medical applications in fields like pharmacodynamic, pharceutical & bio-pharceutical etc. Its mechanism of action and the parameters affecting the release characteristics of drugs were also discussed.
Manoj Kumar Pati
Asst. Prof., EAST, Bhubaneswar
E-mail: manoj.pati09@gmail.com
The history of chitosan (Principal derivatives of chitin) dates back to its first decription of Branconnot in 1811. Rouget later discovered the deacetylated form of chitin, which was called chitosan, in 1859. Chitin, chitosan and its derivatives have received much attention from scientists in different parts of the world. Due to its natural abundance and versatility, many investigations have focused on its properties and various medical applications. Since they are biodegradable non-toxic and bio- compatible, these derivatives have a
potential for use for variety of medical applications. This fact has been further supported from commercialization of many chitin and chitosan based products for clinical uses.
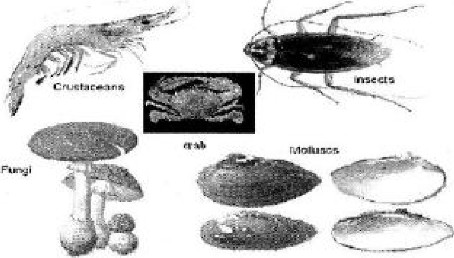
Chitin is widely distributed in marine invertebrates (Fig. 1) insects, fungi and yeast. However chitin is not present in higher plants and higher animals. Generally, the shell of selected crustaceans was reported by Knorr to consist of 30-40% protein, 30-50% calcium carbonate and calcium phosphate and 20-30% chitin. Chitin is
widely available from a variety of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 2
ISSN 2229-5518
source among which the principal
source is shellfish waste such as
shrimps, crabs and crawfish. It also exist
naturally in a few species of fungi.
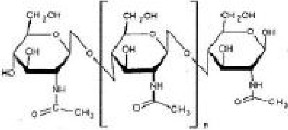
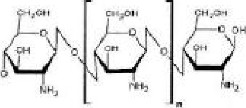
Chitosan (pronounced Ky-toe- san) is derived from a medical called chitin, which is an amino polysaccharide, extracied form the powdered shells of crustaceans like shrimps and crabs. Chitosan is prepared by deacetylation of chitin. To prepare chitin, crab and shrimp shells are demineralized in dilute hydrochloric acid (Hcl), deprotinated in dilute sodium hydroxide (NaoH) and then decolourized in potassium permangate (KMno4). The chitin is then deacetylated to become chitosan by boiling it in a concentrated sodium hydroxide solution as shown in the fig. 2. Biochemical grade/purified chitosan is prepared by repeating the deacetylation process. Pharmaceutical grade chitosan is deacetylated between 90-95% and food grade between 75-80%.
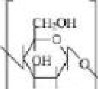
Chitosan is structurally similar to glycosaminoglycans with a chemical formula of (C6H11O4N), schematically represented in Figure 3. Chitosan is a
weak base with a pKa of about 6.2-7.0, and it requires a certain amount of acid to become soluble, and has shown promising characteristics as a modified drug delivery polymer. The word
„chitosan" refers to a large number of
polymers, which differ in their degree of N-deacetylation (40-98%) and molecular weight 50000 - 20000000 Daltons). These two characteristics are very important to its physicochemical properties and may have a major effect on the biological properties. Chitosan salts are soluble in water; the solubility depends on the degree of deacetylation and the pH of the solution. The pharmaceutical requirements of chitosan are: particle size <30µm, density between 1.35 and
1.40 g/cm3, pH 6.5-7.5, insoluble in water, and partially soluble in acids. The chemical and biological properties of chitosan are summarized in Table 1.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 3
ISSN 2229-5518
O-/N-carboxymethylchitosan
(CM-chitosan) is one of the most investigated derivatives of chitosan, obtained under controlled reaction conditions with sodium monochloroacetate. This amphoteric polyelectrolyte has attracted considerable interest in a wide range of medical applications, such as wound dressings, artificial bone and skin, bacteriostatic agents, and blood anticoagulants, due to its unique chemical, physical, and biological properties, especially its excellent biocompatibility. The presence of both This method results in regioselective carboxymethylation of the amino group, so the product of reaction is a well-defined derivative. Several N- carboxyalkylated chitosans were prepared via Schiff base formation from carboxylic acids having aldehyde or keto groups. The resulting carboxyalkylated derivatives find applications as medical materials and fungistatic agents. O-carboxymethy lchitosan is also used to develop a water-soluble matrix polymer for controlled drug release. OCM-chitosan microspheres containing antibiotic drug pazufloxacin mesilate were prepared by the emulsion method and successively
crosslinked with glutaraldehyde.
Chemical modification of the amino and hydroxyl groups of chitosan
carboxyl groups and amino groups in CM-chitosan macromolecules elicits special physicochemical and biophysical properties. It is interesting for pharmaceutical applications because of their novel properties, especially for controlled or sustained drugdelivering systems. Ncarboxymethylation of chitosan is effected through Schiff base formation from the free amino group of chitosan with an aldehyde or keto group and the successive reduction with cyanoborohydride or sodium borohydride.
with sulphate can generate products for pharmaceutical applications. Sulfonation reactions of polysaccharides can give rise to a structural heterogeneity in polymer chain, but on the other hand some structures that emerge from random distribution can reveal good features for biological functions. Sulphated chitosans, that represent the nearest structural analogues of the natural blood anticoagulant heparin, show anticoagulant, antisclerotic, antitumor and antiviral activities. Chitosan derivatives having N- and/or Osulphate groups either alone or in conjunction with other substituents have been widely examined as potential heparinoids. Chitosan sulphates can be synthesized by sulfation of low molecular weight chitosan (Mw 9000–
35,000 Da). Here oleum is used as
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 4
ISSN 2229-5518
sulfating agent and dimethylformamide as medium and demonstrated that chitosan sulphates with reduced molecular weight show a regular increase of anti-coagulant activity, like heparins. Holme et al. converted amino groups of chitosan, with low N-acetyl content, into anionic centers through N- sulfation. They used trimethylamine- sulfur trioxide, which is known to effect selective N-sulfation of amino alcohols. Selective O-sulfonation of chitosan was performed by Zhang et al. They prepared Nalkyl-O-sulphate chitosan by treating N-octyl-chitosan with DMF and chlorosulphonic acid. The thermal stability of such N-alkyl-O-sulphated chitosan decreased with respect to that of the original chitosan. The introduction of substituents into polysaccharide structures disrupt the crystalline structure of chitosan, especially due to loss of hydrogen bonding. N-Alkylsulphate chitosan has an amphiphilic character due to the presence of hydrophobic moieties, alkyl chains, and hydrophilic moieties, sulphate groups. Because of this, it has the capacity to form micelles in water
overdosed drugs selectively and rapidly, and reduce their free blood concentration to a safe level, were developed. Chitosan can be modified with succinic anhydride in order to obtain a water soluble polymer, N succinyl chitosan. The reaction,
described by Aiedeh et al., is performed
and can be used as a potential drug carrier.
A variety of acylation reactions of chitosan are possible by using different acylating agents, such as aliphatic carboxylic acid chlorides (hexanoyl, dodecanoyl and tetradecanoyl chlorides), cyclic anhydrides, cyclic esters. The acylation reaction is not regioselective., Oacylated chitosans were prepared with acyl chlorides in methanesulfonic acid the derivatives of
4-chlorobutyl and decanoyl chlorides showed higher fungidal activities than chitosan. Selectively N-acylated chitosan have been obtained by Lee et al. with butanoic, hexanoic and benzoic anhydride under homogeneous conditions in the presence of methanol. Such a chemical modification of chitosan was carried out to induce a hydrophobic nature to the hydrophilic chitosan backbone and to prevent particle aggregation. Chitosan nanoparticulate systems for intravenous administration, with an assumption that engineered nanoparticle systems can absorb
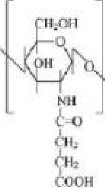
under homogeneous conditions in presence of pyridine and lead to a high degree of chitosan succinylation. The succinylation reaction (illustrated in figure 4) consists of a condensation reaction between the polysaccharide amine group and the electrophilic
carbonyl group of the anhydride. The
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 5
ISSN 2229-5518
reaction involves the formation of an amidic bond with opening of the anhydride ring:
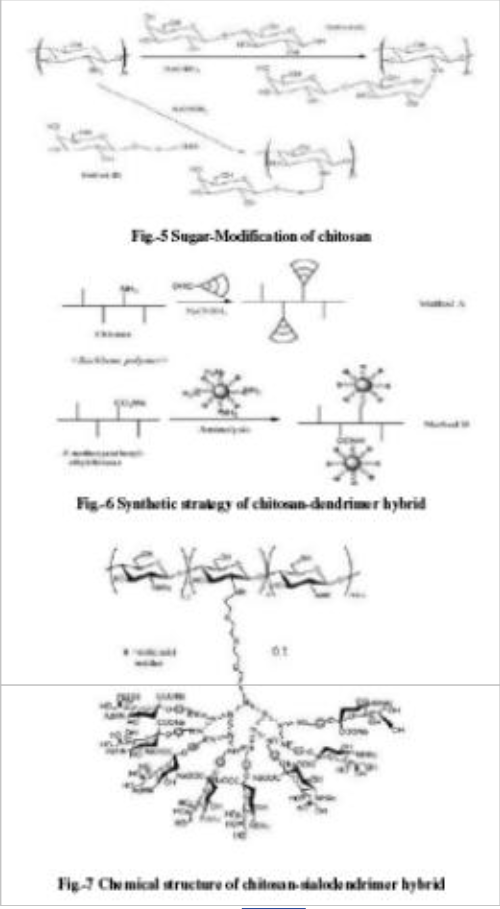
The first report on the modification of chitosan with sugars was by Hall and Yalpani. They synthesized sugar-bound chitosan by reductive N-alkylation with NaCNBH3 using either an unmodified disaccharide (figure 5, method A) or a monosaccharidealdehyde derivative (figure 5, method B). This type of modification has generally been used to introduce cell-specific sugars onto chitosan.
Following this route, Kato et al. prepared lactosaminated N-succinyl- chitosan and its fluorescein thiocarbanyl derivative as a liver-specific drug carrier in mice through a sialoglycoprotein receptor. Galactosylated chitosan prepared from lactobionic acid (LA) and chitosan with 1-ethyl-3- (3- dimethylaminopropyl)-carbodiimide (EDC) and hydroxysuccinimide (NHS) showed promising as a synthetic extracellular matrix for hepatocyte attachment. Yang et al. prepared the chitosan derivatives through the
bearing aldehyde and spacer are synthesized, and then these are reacted
reductive Nalkylation of chitosan as described by Sashiwa and Shigemasa with various mono- and disaccharides. NAlkylated chitosan effectively showed solubility at neutral and basic pH region. Moreover, some derivatives substituted with disaccharides including lactose, maltose and cellobiose showed the solubility at all pH range. These derivatives will overcome the application limit of chitosan represented by its reduced solubility.
Dendrimers are attractive macromolecules owing to their multifunctional properties and useful applications as viral and pathogenic cell adhesion inhibitors. Increasing scientific efforts have gone into the design and synthesis of dendrimers. Dendronized polymers, on the other hand, are also attractive because of their rod-like conformation and nanostructure. Although, several investigations have been published toward the synthesis of dendronized polymers very few reports are available on dendronized polysaccharides, especially related to chitin and chitosan backbone. Sashiwa et al. established at first the synthesis of a variety of chitosandendrimer hybrids mainly by two procedures (Figure 6). In method A, the corresponding dendrimers
with chitosan by reductive N-alkylation.
This procedure is advantageous because
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 6
ISSN 2229-5518
no crosslinking takes place during the reaction. However, generation of reactive dendrimer is limited owing to its steric hindrance. It is possible to generate more reactive dendrimers following method B, which uses commercial amino-dendrimers such as poly (amidoamine) (PAMAM) and poly(ethylene imine) (PEI) dendrimers.
However, method B suffers from the possibilities of cross-linking. The typical example of a “tree-type” hybrid generated by method A is shown in Figure 7. The terminology “tree-type hybrid” is based on the assumption that chitosan is a trunk, the spacer part is a main branch, dendrimer is a subbranch, and the functional sugar is a flower (or leaf). In this case, tetraethylene glycol was modified in 5 or 7 steps to synthesize the scaffold of dendrimer.
PAMAM dendrimers of generation (G) from 1 to 3 bearing tetraethylene glycol spacer were prepared, attached to sialic acid by reductive N-alkylation, and finally attached to chitosan. The degree of substitution (DS) of dendrimer decreased with increasing generation as
0.08 (G = 1), 0.04 (G = 2), and 0.02 (G = 3)
owing to the steric hindrance of
dendrimer. Figure 8 shows the different types of chitosandendrimer hybrids. Sialic acid dendron bearing a focal aldehyde end group was synthesized by a reiterative amide bond strategy. Trivalent (G = 1) and nonavalent (G = 2) dendrons having gallic acid as the branching unit and triethylene glycol as the spacer arm were prepared and initially attached to a sialic acid pphenylisothiocyanate derivative. The focal aldehyde sialodendrons were then convergently attached onto chitosan. The DS of sialodendrimer were 0.13 (G =
1) and 0.06 (G = 2).
The intriguing properties of chitosan have been known for many years and this polycationic polymer (in acidic environments) has been used in the fields of agriculture, industry and medicine. In agriculture, chitosan has been described as a plant antiviral, an additive in liquid multi-component fertilizers and it has also been investigated as a metalrecovering agent in the mining industry.
Chitosan oligosaccharide application leads to blood low-density lipoprotein (LDL)- cholesterol decrease. At the same time the content of the anti- atherogenic fraction of cholesterol, high-
density lipoprotein (HDL)-cholesterol,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 7
ISSN 2229-5518
increases. In general, chitosan oligosaccharide promotes a decrease in cholesterol in the liver. Unlike high- molecular weight chitosan, chitosan oligosaccharide application does not lead to compensatory cholesterol
Chitosan oligosaccharide is a cation by its chemical nature. Therefore, it binds blood chlorine ions. Chlorine ions stimulate angiotensin-converting enzyme activation thus affecting the renin-angiotensin system. Chitosan oligosaccharide stimulates production of nitrogen oxide (a strong vasodilator).
6.1.3 Immunity
Immune-stimulating function of chitosan oligosaccharide is conditioned by the similarity of its molecular structure to that of cell membrane. Chitosan oligosaccharide has a favourable impact on humoral and cell immunity. It stimulates production of interferon and interleukin-1. Chitosan oligosaccharide also has a positive stimulating effect on Blymphocytes, neutrophils and macrophages. The preparation is effective in cases of various diseases accompanied by immunity disorders.
Chitosan oligosaccharide prevents development of fatty liver caused by the action of hepatotrope
synthesis enhancement. Furthermore, when chitosan oligosaccharide gets into the intestine it does not promote excretion of fat-soluble vitamins and microelements from the organism as compared to high-molecular chitosan.
poisons. It decreases production of TNF- a, which is a factor of liver fibrosis and cirrhosis, stimulates hepatocyte growth factor (HGF), interleukin-2 production, which are the factors of liver normal regeneration. It is used in cases of chronic hepatitis, liver cirrhosis, fatty liver, and alcohol hepatosis, toxic liver damage.
Chitosan oligosaccharide prevents alcohol overdose symptoms such as vomiting, headache, diarrhoea; alcohol reduced neurological disorders such as amnesia, coordination disorders and attention derangement. It also reduces alcohol intoxication symptoms to shorter time periods.
Chitosan oligosaccharide is the source of glucosamine, which stimulates chondroitin sulphate, and glucosamineglycans of hyaluronic acid production.It prevents aggressive behaviour of dystrophic-degenerative joint diseases.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 8
ISSN 2229-5518
Chitosan oligosaccharide stimulates fibroblasts production by means of affecting fibroblasts growth factor (FGF). Thus, collagen production is stimulated as well as other components of connective tissue. The
Chitosan oligosaccharide suppresses suprogenous micro-flora growth; at the same time it stimulates
Due to its unique polymeric cationic character and its gel and film
development of drug delivery systems. It is presently considered as a novel carrier material in drug delivery systems, as indicated by the large number of studies published over the last few years. High-purity chitosan has been considered for pharmaceutical formulation and drug delivery applications in which attention has been focused on its absorption–enhancing, controlled drug release and bio- adhesive properties. Medical and pharmaceutical applications of chitosan include drug delivery, wound healing ointments and dressings, artificial skin, homeostatic agents, enzyme immobilization, dialysis membranes, contact lenses or eye bandages, orthopaedics, surgical sutures and dentistry. Since chitosan exhibits favourable biological properties such as non-toxicity, biocompatibility and
biodegradability, it has attracted much
preparation promotes acceleration of the woundhealing process, and connective tissue gets an ordered structure. Chitosan oligosaccharide application prevents rough scar formation.
bifido- and lacto-bacteria reproduction. Chitosan oligosaccharide application results in dispersion phenomena and meteorism (tympanism) decrease.
forming properties, chitosan has been extensively examined in the pharmaceutical industry for its potential in the interest in pharmaceutical research as a polymeric drug carrier and as a novel drug absorption enhancer across nasal and intestinal epithelia. The ability of chitosan to enhance the paracellular transport of several peptide drugs, both in vivo and in vitro, is considered to be the most important pharmaceutical application of chitosan. The use of chitosan in the development of drug delivery preparations is based on studies with chitosan intra-gastric tablets and chitosan–coated drug delivery systems. Drugs dispersed in chitosan were released at a constant rate, thus highlighting its potential as a sustained-release matrix polymer. Kneading of low-molecular weight chitosan with several of poorly soluble drugs increased their dissolution.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 9
ISSN 2229-5518
The bioavailability of drugs has been improved by the use of muco- adhesive dosage forms. This is because by prolonging the residence time of drug carriers at the absorption site, improved absorption of drugs can be achieved. It is acknowledged that higher molecular weight chitosan possesses better muco-adhesion as compared to lower molecular weight chitosans. Insulin– loaded liposomes coated with chitosan were developed to improve the enteric absorption of insulin. The results of the above-mentioned study indicated that as chitosan concentration increased in the coating, the muco-adhesion improved, resulting in an increased absorption of insulin. This mucoadhesive property makes chitosan an ideal candidate for oral delivery of drugs.
The development of new carrier systems for gene delivery represents an enabling technology for treating many suspensions during in vivo
studies were investigated and showed a
significant increase in ocular drug bioavailability. Through the investigation of several ocular drug delivery studies it was apparent that chitosan-loaded nanoparticles and microspheres displayed good ocular tolerance. In addition to its muco- adhesive properties, chitosan is effective
in retarding the rate of drug release and
genetic disorders. Since, a critical barrier to successful gene therapy remains in the formulation of an efficient and safe delivery vehicle, non-viral delivery systems have been increasingly proposed as alternatives to viral vectors owing to their safety, stability and ability to be produced in large quantities. Chitosan not only increases transformation efficiency but also through the addition of appropriate ligands to the DNA-chitosan complex, facilitates more efficient gene delivery via receptormediated endocytosis without cytotoxic effects.
The poor bioavailability of topically applied ophthalmic drugs implies a necessity for frequent instillation to achieve therapeutic effect. This inconvenience could be overcome by a prolonged release of the drug in the corneal area. The use of chitosan– based colloidal
represents a useful approach to increase ocular bioavailability of drugs.
Effective nasal drug delivery depends on increased absorption of the drug through the nasal mucosa without undesired side–effects. Several studies have been conducted to increase the bioavailability of compounds by means
of co-administration of various
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 10
ISSN 2229-5518
absorption enhancers including surfactants, bile salts and cyclodextrins. However, a nasal formulation with improved absorption of macromolecules is still a challenge because of the short retention time in the nasal cavity due to the efficient physiological clearance mechanism. Chitosan may be a good option in nasal drug delivery as it binds to the nasal mucosal membrane with an increased retention time and is therefore potentially a good absorption enhancer for nasal drug delivery.
In controlled release technology, biodegradable polymeric carriers offer potential advantages for the prolonged release of low–molecular weight compounds. The susceptibility of chitosan to liposome makes it biodegradable and an ideal drug carrier. Molecules such as bovine serum albumin, diphtheria toxoid and bisphosphonates have been successfully incorporated into chitosan microspheres. Coating of chitosan microspheres with paraffin oil or poly (D-lactic acid) polymer, the initial burst effect was controlled and drug release was modulated. Pharmacokinetic and
8. References
tissue–distribution studies were performed in mice using fluorescent glycol chitosan and N–succinyl– chitosan. Both chitosans demonstrated a good retention in the blood circulation and a slight accumulation in tissues, suggesting that chitosan is an effective carrier for drugs that are excreted rapidly.
After the successful extraction of chitosan from chitin it has remain the polymer of first choice for various medical applications like controlled drug delivery, gene delivery, ocular drug delivery and for the treatment of wounds and burns etc. Hence keeping the importance of this polymer in view the review emphasizes most recent papers published regarding the synthesis of different kinds of derivatives of chitosan and their applications in different medical fields. We include an extensive bibliography of recent studies, both basic and applied. So going through the applications of chitosan for the beneficial of human being it is not surprising at all to call it a “Wonder Polymer”.
1. Aiedeh, K.; Taha, M.O, (1999) Journal of Arch. Pharm 332, 103–107.
2. Alban, S., Schauerte, A., Franz, G., (2002) Journa of Carbohydrate Polymer 47, 267–
276.
3. Allan, C.R. and Hadwiger, L.A. (1979) Exp. Mycol., 3, 285-287.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 11
ISSN 2229-5518
4. Badawy, M.E.I., Rabea, E.I., Rogge, T.M., Stevens, CV., Smagghe, G., Steurbaut, W., Hofte, (2004) M., Biomacromolecules 5, 589–595.
5. Biagini, G., Bertani, A., Muzzarelli, R.A.A., Damadei, A., Di Benedetto, G., Belligolli, A., Riccoti, G., Zucchini, C., Rizzoli, (1991) C., Biomaterials 12, 281–286.
6. Bosman, A. W., Janssen, H. M., Meijer, E. W., (1999) Chem. Rev., 99, 665.
7. Chen, S.C., Wu, Y.C., Mi, F.L., Lin, Y.-H., Yu, L.-C., Sung, H.-W., (2004) J. Control.
Release 96, 285 –300.
8. Chen, Y., Tan, H. M., (2006) Carbohydr. Res 341, 887–896.
9. Desai, U. R., (2004) Med. Res. Rev 24, 151–181.
10. Dodane, V., & Vilivalam, V.D., (1998) September Pharmaceutical Sciences and
Technology Today, 1(6), 246-253.
11. Drozd, N. N., Sher, A. I., Makarov, V. A., Vichoreva, G. A., Gorbachiova, I. N., Galbraich, L. S., (2001) Thromb. Res 102, 445–455.
12. Frechet, J. M. J., (1994) Science, 263, 1710.
13. Hall, L.D., Yalpani, M., (1980) J. Chem. Soc. Chem. Comm. 1153–54.
14. Hamman, J.H., Stander, M., Junginger, H.E., & Kotze, A.F., (2000) STP Pharma
Sciences, 10(1), 35-38.
15. Hejazi, R., & Amiji, M., (2003) Journal of Controlled Release, 89, 151-165.
16. Holme, K. R., Perlin, A. S., (1997) Carbohydr. Res. 302, 7–12.
17. Horton, D., and Just, E.K., (1973) Carbohydr Res. 29, 173– 180.
18. Huang, R.H., Du, Y., Zhang, L.S., Liu, H., Fan, L. H., (2004) React. Funct. Polym. 59,
41–51.
19. Issberner, J., Moors, R., Vogtle, F., Angew. Chem., (1994) Int. Ed. Engl., 33, 2413.
20. Jiang, D.-L., Aida, T., (1998) Kobunshi 47, 812.
21. Kato, Y., Onishi, H., Machida, Y., (2001) J. Control. Release 70, 295–307.
22. Kitov, P. I., Sadowska, J. M., Mulvey, G., Armstrong, G. D., Ling, H., Pannu, N. S., Read, R. J., Bundle, (2000) D. R., Nature, 403, 669.
23. Knorr, D. (1984) Food Technol.., 38(1), 85-97.
24. Kurita, K., (2006) Marine Biotechnol. 8, 203–226.
25. Lee, D.-W., Baney, R. H., (2004) Biotechnol. Lett. 26, 713–716.
26. Lee, D.-W., Powers, K., Baney, R., (2004) Carbohyd. Polym. 58, 371–377.
27. Liu, Y.-F., Huang, K.-L., Peng, D.-M., Ding, P., Li, G.-Y., (2007) Int. J. Biol.
Macromol. 41, 87–93.
28. Malenfant, P. R. L., and Frechet, J. M. J., (2000) Macromolecules, 33, 3634.
29. Muzzarelli, R.A.A., (1988) Carbohydr. Polym., 8, 1–21.
30. Muzzarelli, R.A.A., Muzzarelli, C., Tarsi, R., Miliani, M., Gobbanelli, F., Cartolari, M., (2001) Biomacromolecules 2, 165–169.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 12
ISSN 2229-5518
31. Muzzarelli, R.A.A., Tanfani, F., Emanuelli, M., Mariotti, S., (1982) Carbohydr. Res.
107, 199–214.
32. Park, I.K., Yang, J., Jeong, H.J., Bom, H.S., Harada, I., Akaike, T., Kim, S., Cho, C.S., (2003) Biomaterials 24, 2331–2337.
33. Paul, W., & Sharma, C.P., (2000) S.T.P. Pharma Sciences, 10(1), 5-22.
34. R.A.A. Muzzarelli, (2005) Adv Polym Sci., 186, 151–209.
35. Reuter, J. D., Myc, A., Hayes, M. M., Gan, Z., Roy, R., Qin, D., Yin, R., Piehler, L. T., Esfand, R., Tomalia, D. A., Baker, J. R., (1999) Jr. Bioconjugate Chem., 10, 271.
36. Rinaudo, R., Desbrieres, J., Le Dung, P., Binh, T., Dong, N.T., (2001) Carbohydr.
Polym. 46, 339–348.
37. Sashiwa, H., Kawasaki, N., Nakayama, A., Muraki, E., Yamamoto, N., Zhu, H., Nagano, H., Omura, Y., Saimoto, H., Shigemasa, Y., Aiba, S., (2002) Biomacromolecules 3, 1120–1125.
38. Sashiwa, H., Kawasaki, N., Nakayama, A., Muraki, E., Yamamoto, N., Aiba, S., (2002) Biomacromolecules, 3, 1126– 1128.
39. Sashiwa, H., Shigemasa, Y., and Roy, R., (2002) Carbohydr. Polym., 47, 191.
40. Sashiwa, H., Shigemasa, Y., and Roy R., (2001) Macromolecules 34, 3211.
41. Sashiwa, H., Shigemasa, Y., and Roy, R., (2000) Macromolecules, 33, 6913.
42. Sashiwa, H., Shigemasa, Y., and Roy, R., (2001) Macromolecules, 34, 3905.
43. Sashiwa, H., Shigemasa, Y., (1999) Carbohyd. Polym. 39, 127– 138.
44. Schluter, A. D., Rabe, J. P., Angew. Chem., (2000) Int. Ed. Engl., 39, 864.
45. Shigemasa, Y., Ishida, A., Sashiwa, H., Saimoto, H., Okamoto, Y., Minami, S., (1995) Matsuhashi., Chem. Lett 24, 623–624.
46. Sui, W., Wang, S., Chen, G., Xu, G., (2004) Carbohydr. Res 339, 1113–1118.
47. Thanou, M., Nihot, M.T., Jansen, M., Verhoef, J.C., Junginger, H.E., (2001) J. Pharm.
Sci. 90, 38–46.
48. Tolaimate, A., Desbrieres, J., Rhazi, M., Alagui, M., Vincendon, M., Vottero, P., (2000) Polymer, 41, 2463-2469.
49. Tomaria, D. A., Naylor, A. M., Goddard W. A., (1990) III Angew. Chem. Int. Ed.
Engl. 29, 138.
50. Underhill, R. S., Jovanovi, A. V., Carino, S. R., Varshney, M., Shah, D., Dennis, D.
M., Morey, T. E., Duran, R. S., (2002) Chem. Mater. 14, 4919–4925.
51. Vetter, S., Koch, S., Schluter, A. D., (2001) J. Polym. Sci., A: Polym.Chem., 39, 1940.
52. Vikhoreva, G., Bannikova, G., Stolbushkina, P., Panov, A., Drozd, N., Makarov, V., Varlamov, V., Gal"braikh, L., (2005) Carbohydr. Polym. 62, 327–332.
53. Warner, D.T., Coleman, L.L., (1958) J. Org. Chem. 23, 1133–1135.
54. Yalpani, M., Hall, L.D., (1984) Macromolecules 17, 272–81.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 13
ISSN 2229-5518
55. Yang, T.C., Chou, C.C., Li, C.F., (2002) Food Res. Inter. 35, 707– 713.
56. Yang, T-C., Chou, C.-C., Li, C.F., (2005) Int. J. Food Microb 97, 237– 245.
57. Zeng, F., Zimmerman, S. C., (1997) Chem. Rev., 97, 1681.
58. Zhang, C., Ping, Q., Zhang, H., Shen, J., (2003) Carbohydr. Polym. 54, 137–141.
59. Zhu, A.P., Liu, J.H., Ye, W.H., (2006) Carbohydr. Polym. 63, 89–96.
60. Zubarev, E. R., and Stupp, S. I., (2002) J. Am. Chem. Soc., 124, 5762.
IJSER © 2013 http://www.ijser.org
CNJ ):aum.aldSctc d. re:R d\ V'al.anw4:b:w t.J lt:lll

lSSNlll,.!Dta
Crab/Shrimp shells
!
Deuriuerlizatiou (dilutt HCL)
!
Deproleinatiou (dilulNaOH)
!
Dcolouri:zanon (ICMn0 4)
!
Chitin
!
D cetyulatiou (concemrated aOH)
!
Chitosan
tlg.-l ChitOMll l'rodu t'tion Aow Oi ugrom
Cl.....,..l JllOJl'ftlel orCilJio... | Bio ical'"oper""d<t>ir-. |
C'..nbopoJpt!nan.; | 8tOK" fi,lc- |
llidlor;<d <lcmirv m pH 6 5 | Nonulpo!Yua |
Aclate1-u lo t1e1.1li\'dy cd $ud'ac:u | Dw d.bk to llllll'DWbil ody olUtrtUa• |
knn ek 'A'ilh fO}pmon | Saio and nontoU |
Ha»11. t.r """ liO<ru pol) eecuoi)1< | HC'JD0)1•1il:. biKitfioSotic IJ.Ud ii•.-i tltil: |
Vi . .• blgb to lov.· CbehU i c.?' tan L O!otflO'Ilsl ooe·ak | Spm:uc..tol .\u1-t:&.tr;m)j:!ell |
Anlulbk 10 dkulkll JDX:hfiadao | AJJ:i-<holc CJCJllic |
Lt>"1ive amino hydtoX)-i FOUP1 | v ik |
'f abSt I:Ou:mic:.S :l.Dd biolosieal proputiu or <'hitonn
![]()
International Journal of Scientific & Engineering Research Volume 4, Issue 1, Januaty-2013 15
ISSN 2229-5518
![]()
H
![]()
![]()
![]()
![]()
![]()
..
NH
"
![]()
IJSER ©2013
http /lwww! ser orq
International Journal of Scientific & Engineering Research Volume 4, Issue 1, January-2013 16
ISSN 2229-5518