International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2674
ISSN 2229-5518
Chemical and Biochemical Mechanistic Fate of Acephate
Vijay Kumar, Niraj Upadhay
Abstract— Acephate belongs to a hefty assembly of organophosphorus pesticides, well-known as inhibitors of acetylcholinesterase activity. Acephate and other organophosphorus pesticides have been extensively used in world agriculture to manage insect or pests of a number of economically important crops. Acephate is not restricted to anticholinesterase action, but comprise genotoxicity and teratogenicity. Such severe health consequences signify a requirement for a better understanding of the fate of acephate in the environment. The safe and effective use of pesticides requires knowledge of their mode of action in pests and adverse effects in non-target organisms coupled with an understanding of their metabolic activation and detoxification; keeping these things in mind we wrote a review on acephate. In this review we have studied the mechanistic pathway of toxicity of acephate, mode of action, different methods of decomposition viz. acidic, neutral and basic hydrolysis, decomposition in soil, decomposition by mammals and decomposition in plants.
Index Terms— Acephate, Acetylcholinesterase, Decomposition, genotoxicity, Hydrolysis, methamidophos, Organophosphorus Pesticides,
1 INTRODUCTION
—————————— ——————————
here by we are going to highlight the mechanistic pathway of
he conventional crop security events include the use of pesticides that are toxic to non-targeted organisms; cur- rently the pesticides based contamination is the mammoth
and significant risk to the environment and non targeted or- ganisms ranging from beneficial soil microorganisms, insects,
plants, fish and birds [Md. Wasim et al. 2009].
Trace contamination of pesticides present in the environ- ment creates a lot of pollution dilemma due to their toxicity and bioaccumulation property. Pesticides are used in agricul- ture, medicine, industry and the household [Md. Wasim et al.
2009]. Acephate (O,S-dimethyl acetylphosphoramidothioate) is a racemic organophosphorus insecticide used as broad spec- trum insecticide for foliar treatment of vegetable, fruit and field crops, cotton, commercial ornamentals, and in around

poultry houses and dairies [WHO Report, 2002].
toxicity and decomposition.
2 TOXICITY
Studies revealed that acephate and methamidophos soluble in polar solvents and solubility increased with temperature [Geen, et al. 1981; Wang, et al. 2007; Yen, et al. 2000] hence life time, persistence and leaching of both acephate and methamidophos increase with polar solvent. The basic mode
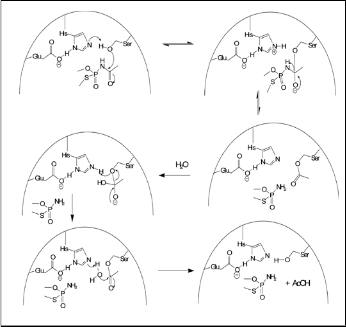
Figure 1: Structure of Acephate and Methamidophos.
Acephate is not constrained to anticholinesterase action, but include genotoxicity and teratogenicity. Such severe health consequences signify a requirement for a superior understand- ing of the fate of acephate in the environment (Hussain, 1987; Geen, et al. (1981). Acephate itself considered toxic but after the decomposition to methamidophos become more toxic, so
————————————————
• Vijay Kumar is currently pursuing doctrate degree program in chemistry at Lovely Professional University, India.
• Corresponding Author -Dr. Niraj is currently worked as associate professor
(chemistry) at Lovely Professional University, India.
contact number +919888897250, E-mail: vijay.kumar8491@yahoo.in
Figure 2: Protection of AChE from Aging by Acephate.
of action of acephate is considered like the other organophosphate pesticide’s mode of action that is inhibition of acetylcholinesterase (AChE) activity, but acephate inhibits the AChE in different way. The selective toxicity of acephate is considered to be due to facile conversion to methamidophos (fig 2). Acephate itself acts as a weak cholinesterase inhibitor
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2675
ISSN 2229-5518

but inhibited brain cholinesterase nine times more it did than
Human Plasma | Bovine RBC |
| μg/ml | I50, Molar | μg/m l | I50, Molar |
Acephate | >500 | >2.7 X 10-3 | >500 | >2.7 X 10-3 |
Methamid ophos | 23 | 1.6 X 10-4 | 4.3 | 3.1 X 10-5 |
Table 1: Acephate and Methamidophos toxicity of Human
Plasma and Bovine RBC.
erythrocyte cholinesterase, both insecticides have similar insecticidal potency, but different mammalian toxicity i.e. methamidaphos > acephate [Maul, et al. 2004; Md. Mahajna, et al. 1997; White et. al. 1998].
Acephate is moderately toxic and methamidophos is highly toxic and acephate became toxic after twelve hours after its
use due of optical nature [Miyazaki, et al. 1988]. Acephate has greater affinity than does methamidophos for the AChE active site, so acephate pre-exposure provided protection against the inhibition of RBC and brain acetylcholinesterase (AChE), and plasma cholinesterase (ChE) activities in rats exposed to both acephate and methamidophos [Singh 1986].
So we can say that initially acephate acts as weak AChE inhibitor and strong masking agent for AChE inhibition. As per the WHO report 2002, we revealed that the species sensitivity for inhibition of brain cholinesterase by acephate was in order to; rat > cynomolgus monkey > human, that for inhibition of erythrocyte cholinesterase was rat > cynomolgus monkey = human, while that for inhibition of plasma cholinesterase was the reverse, human > cynomolgus monkey
> rat (table 1). In human acephate is quickly absorbed through the skin, lung and gastrointestinal tract and are broken down in the liver, breakdown products are readily excreted via respiration and urine (Chuckwudebe, et al. (1984). Toxicity of acephate to kidney cells has been mediated at least in part through a free radical mechanism involving lipid peroxidation and those two antioxidant inhibitors of lipid peroxidation, desferrioxamine and 2-methylaminochroman, reduce acephate-induced renal tubular cell injury (Poovala, et al. (1998); Datta, et. al. (2010).
It has been concluded regarding the effect of acephate on testicular functions of Albino Rats, acephate affect semen qual- ity in adult males (Albino Rats) and reproductive health of male mice either directly or indirectly via interference with endocrine status [Joshi and Sharma 2012; Farag, et al. 2000; Rattner and Hoffman, 1984]. Acephate exposure has been re- sulted in the inhibition (dose dependent manner) of both Mg2+ and Na+- K+ ATPase activity in all brain regions studied by the alterations in The Mg2+ ATPase activity and Na+- K+ ATPase in
Cerebral Cortex, Hippocampus, Cerebellum and Medulla ob- longata of control and experimental rats [Mohiyuddin, et al.
2010].
2.1. Mechanistic Approach of Acephate
From the above discussion the straight forward conclusion has been drawn that is acephate act as masking agent for AChE [Singh 1986], but only for few hours, once broken into methamidophos, it become highly toxic [Miyazaki, et al. 1988]. Mechanistic point of views, firstly acephate react with AChE and cleave AcOH moiety as like common mechanism of ace- tylcholine. The basic mechanism of AChE inhibition with acephate is analogous to the reaction of enzyme with ACh, excluding for the reaction in which the leaving group of the acephate is lost, so the enzyme becomes phosphorylated in- stead of acetylated. Phosphorylation is a two-step addition- elimination reaction in which the addition step is rate- determining, while the elimination process is faster. It should be noted that phosphorylation occurs via a trigonal bipyramidal intermediate, whereas in the case of acetylation is anticipated through a tetrahedral intermediate. The irreversibly inhibited phosphorylated enzyme can no longer hydrolyze acetylcho- line. This leads to an accumulation of acetylcholine in cholin- ergic receptors and consequent continuous stimulation of the nerve fiber [Kibong, et al., 2011]. Phosphorylated AChE hav- ing the stable bonding and forces than acetylated one, but it can undergo a possible secondary process. Among these first one is the reactivation-hydrolysis of phosphorylated enzyme. But the rate of hydrolysis is much slower than in the case of an acetylated enzyme.
Figure 3: Inhibition and “Aging” of AChE with
Methamidophos.28
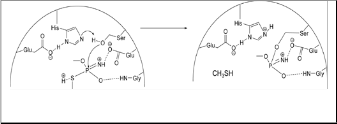
Another mechanism is the breaking of the PO-C bond the inhibited enzyme known as “aging” [Kibong, et al. 2011]. In present case once acephate decomposition product methamidophos inhibits the AChE (fig. 3) and block the further hydrolysis [Singh 1986].
3 Decomposition
3.1 Hydrolysis
Hydrolysis either acidic or basic considered as the basic de- composition method for organophosphates. Acephate and methamidophos do not reveal pH dependent solubility in aqueous and in organic solvents. The P-N bond of methamidophos was hydrolyzed at pH 2, but not of acephate. Thus, the presence of an electron rich domain stabilizes acephate's P-N bond. The CH3S-P bond of both insecticides was equally hydrolyzed at pH 11 [White, et al. 1998]. Mecha- nisms of alkaline hydrolysis of acephate associated to mecha-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2676
ISSN 2229-5518
nism of alkaline hydrolysis is followed by P−N bonds are cleaved according to the multistep addition−elimination scheme, whereas the breakage of P−O and P−S bonds appears to be consistent with the one-step direct displacement mecha- nism [Dyguda-Kazimierowicz, et al. 2008].
3.2 Photocatalytically
The photocatalytic degradation of a commercial methamidophos emulsion in aqueous suspension containing mesoporous titania nanoparticles under UV irradiation was investigated. The mineralization rate of methamidophos went up steadily as prolonging the irradiation time and reached
95% after 4h irradiation based on determination of the end- products of methamidophos [Dai, et al. 2008]. The ZnFe2O4- TiO2 composite photocatalyst has been prepared by sol-gel method and used to degrade acephate successfully. It demon- strates that the photocatalytic degradation of acephate follows pseudo-first-order degradation kinetics (ln(C0/C)=kt) [Weina, et. al. 2012]. Also photocatalytic degradation of acephate in- vestigated in the presence of TiO2, it has been found that the acephate can be degradated and mineralized. Under acidic condition it has been indicated that the decomposition of acephate began from the destruction of C–N and P–N bonds [Han, et al. 2009].
the risk of leaching to the aquatic environment is high if it is not quickly degraded. The acephate degradation rate in air (11 days) > dry soil (16 days) > wet soil (more than 40 days) > wa- ter (more than 80 days). Sandy soils with low microbial activi- ty are more prone to acephate leaching than clayey soils rich in humic matter [Chai, et al. 2010].
It has been invastigated that acephate in the environment can be mineralized to CO2, methyl mercaptan and phosphoric acid through either methamidophos or O-methyl N- acetylphosphoramidate, O, S-dimethyl phosphorothioate and further to CO2, methyl mercaptan and phosphoric acid. Little is known about the microorganisms or enzymes responsible for each step of those pathways. Although studies on acephate degradation in soils have been reported during the last decade only one has identified a bacterium that can initiate the path- way by hydrolysis of acephate [Yen et al. 2000; Szeto, et. al.
1979; Battu, et al. 2009]. It was shown that Hyphomicrobium sp. can degrade several OP compounds, including acephate [Wang, et al. 2010; Nisar, et al. 2009; Pinjari, et al. 2012]. Deg- radation of the acephate by a developed bacterial consortium ER, consisting of the bacterial isolates Exiguobacterium sp. BCH4 and Rhodococcus sp. BCH2. The consortium was capable of degrading acephate up to 75.85%, with an initial concentra- tion of 50 mg L−1 within 6 days at 30 °C under static condi- tions. The effect of various physicochemical parameters, such as pH, temperature, and increasing acephate concentration affects the biodegradation rate of acephate [Swapnil, et al.
2012]. The net decomposition of the acephate by; acidic/basic
hydrolysis, photolytically, mammalians, soil and plants is dif-
ferent (fig. 5).
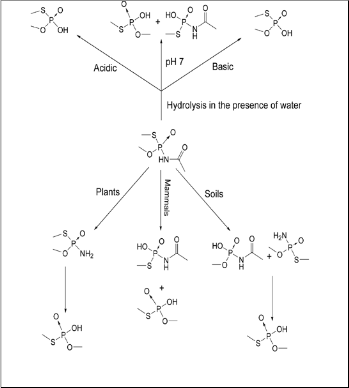
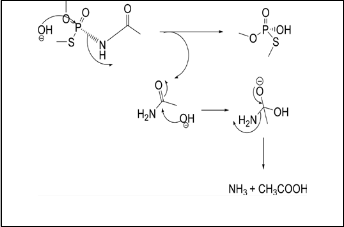
Figure 4: SN2 Mechanistic Pathway of the Basic
Hydrolysis of Acephate.
A cost effective method of decomposition was developed for industrial effluents by using ultrasound in combination of H2O2 at different frequencies, different ultrasonic wave ampli- tudes, pH and concentrations of the solutions. Decomposition of methidathion was effective with ultrasound at greater sound wave amplitude. When the pH was controlled below the pka value of methidathion, significantly better decomposi- tion of methidathion resulted. The decomposition of methida- thion appeared to follow first-order reaction kinetics [Robina, et al. 2008; Hung, et al. 2002].
3.3 Decomposition in Soil
Acephate breakdown in soil to Methamidophos is a kind of organophosphate pesticide that is persists in the environment. Studies have been indicated that degradation of acephate is of first-order kinetics and acephate is poorly sorbed to soil, thus
Figure 5: Decomposition Fate of Acephate under different conditions.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2677
ISSN 2229-5518
A 14C foliar study revealed that acephate more than 50% was absorbed by leaves within 24 hours after their application and considerably more toxic to third stage tobacco bud worms than adult one [Don 1979]. In greenhouse studies regarding the seed application of acephate and soil water content on acephate uptake and phytotoxicity for corn (Zea mays L.) and cotton( Gossypium hirsutum L.) seedlings revealed that, treat- ments exhibited no phytotoxic effects, because acephate nei- ther promoted nor inhibited emergence, nor did it significant- ly affect plant weight. However, plant acephate and methamidophos concentrations were significantly affected by acephate seed-treatment methods. Soil water treatments did not change plant emergence, but plant weight for a given growth stage increased as soil water content was increased. An inverse relationship existed between soil water content and plant acephate and methamidophos concentrations. All acephate seed treatments responded similarly. This result might be attributed to dilution of acephate in large plants grown under increased soil water contents. Plant acephate and methamidophos concentrations varied with growth stage in response to acephate dilution for the given water content. Dif- ferences in methamidophos concentration for a given tissue level of acephate indicated that cotton may be more effective than corn in metabolizing acephate. Potential soil and plant factors, which may have caused differences in acephate up- take, are discussed [Hill, et al. 1984].
Greenhouse studies were conducted from 1996 to 1998 to determine the efficacy of spinosad, and acephate, against western flower thrips (Frankliniella occidentalis Pergande) on transvaal daisy (Gerbera jamesonii H. Bolus ex. Hook f). In addi- tion, the number of natural enemies inside and outside the greenhouse was determined. Studies were arranged in a ran- domized complete-block design with four blocks and four treatments per block. Three rates of spinosad, 50, 100, and 200 mg L–1(ppm), and one rate of acephate, 600 mg L–1 were used in all three studies. Plants were artificially inoculated at bloom with 10 adult western flower thrips. The number of live and dead thrips was counted from each plant. In all three studies, both spinosad and acephate controlled thrips. However, there was more variation in the average number of live thrips for acephate than spinosad across years. In all treatments fewer live thrips and more natural enemies were found on plants outside the greenhouse than inside the greenhouse. This sug- gests that placing plants outdoors allows the natural enemies of thrips to colonize plants and provide supplemental control [Raymond, et al. 2000]. Earthworms are an important part of the soil ecosystem. They help improve soil structure and soil chemical and biological properties acephate and other pesti- cides also affect these farmer friendly organisms (www.cas.psu.edu.).
4 Conclusion
Acephate breakdown Toxicity point of views acephate has greater affinity than does methamidophos for the AChE active site, so acephate pre-exposure provided protection against the inhibition of RBC and brain acetylcholinesterase (AChE), but once acephate decomposed to methamidophos it is highly
toxic. Application of acephate should be restricted or avoided during wet seasons with heavy rainfall and flooded soil as in paddy cultivation. Sandy soils with low microbial activity are more prone to acephate leaching than clayey soils rich in hu- mic matter.
There are number of gaps observed after the broad study of acephate, mode of bonding of acephate (H-bonding and char- acterization), metal based decomposition, nano-material based decomposition, method of detection of acephate, not a broad study about UV-visible, FTIR and NMR based detection of acephate, decomposition in soil, biotechnical decomposition, interaction with soil bacteria, effects of acephate and decom-
posed moieties of acephate (HPO4 and CO2) on soil fertility
3-
and effects on plants growth. As acephate decomposition rate decrease in wet soil so there is need to study more about the effect of acephate on soil organism under wet condition espe- cially earthworms. The major need of the hour is the study regarding the formulation of pesticides, because without the use of pesticides it is very typical to feed fast mounting popu- lation.
5 Acknowledgements
Authors would like to thank UGC for RGNF and Department of Chemistry (Lovely Professional University) for providing necessary library and laboratory facilities.
REFERENCES
[1] Battu R, Sahoo S, Jyot G, “Persistence of Acephate and Cypermethrin on
Cotton Leaves, Cottonseed, Lint and Soil,” Bull. Environ. Contam. Toxicol. 82,
124–128, 2009.
[2] Chai LK, Wong MH, Mohd-Tahir N, Christian H, Hansen B, “Degradation and Mineralization Kinetics of Acephate in Humid Tropic Soils of Malaysia,” Chemosphere 79, 434–440, 2010.
[3] Chuckwudebe AC, Hussain MA, Oloffs PC, “Hydrolytic and Metabolic
Products of Acephate,” J. Environ. Sci. Health. B19, 501-522, 1984.
[4] Dai K, Peng T, Chen H, Zhang R, Zhang Y, “Photocatalytic Degradation and Mineralization of Commercial Methamidophos in Aqueous Titania Suspen- sion,” Environ. Sci. Technol. 42, 1505–1510, 2008.
[5] Datta S, Dhar P, Mukherjee A, Ghosh S, “Influence of Polyphenolic Extracts from Enydra fluctuans on Oxidative Stress Induced by Acephate in Rats,” Food Chem. Toxicol. 48, 2766-2771, 2010.
[6] Don LB, “Fate and efficacy of acephate after application to plants and insects,”
J. Agric. Food Chem. 27, 268–272, 1979.
[7] Dyguda-Kazimierowicz E, Andrzej Sokalski W, Leszczynski J, “Gas-Phase Mechanisms of Degradation of Hazardous Organophosphorus Compounds: Do They Follow a Common Pattern of Alkaline Hydrolysis Reaction As in Phosphotriesterase?,” J. Phys. Chem. B. 112, 9982–9999, 2008.
[8] Earthworms - Penn State College of Agricultural Sciences Research. 2008, available at www.cas.psu.edu.
[9] Farag AT, Eweidah MH, El-Okazy AM, “Reproductive Toxicology of
Acephate in Male Mice,” Reprod.Toxicol. 14, 457-462, 2000.
[10] Geen G H, Hussain MA, Oloffs PC, McKeown BA, “Fate and Toxicity of
Acephate (Orthene) Added to a Coastal B. C. Stream,” J.Environ.Sci.Health. B
16, 253-271, 1981.
[11] Han ST, Li J, Hai-Ling X, Da-Nian X, Zuo Y, Zhang JH, “Photocatalytic De- composition of Acephate in Irradiated TiO2 Suspensions,” J. Hazard. Mat. 163,
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 2678
ISSN 2229-5518
1165–1172, 2009.
[12] Hill RL, Dalili B, Cruse RM, Felsot AS, “Effect of Seed-Treatment Methods and Soil Water Content on Early Growth and Acephate Uptake by Corn and Cotton Plants,” J. Econ. Entomol. 77, 880-884, 1984.
[13] Hung DQ, Wohlers J, Thiemann W, “The Mineralisation of Methamidophos using Ionised AN Air Water Treatment Pilot System and Ultraviolet Irradia- tion,” Water Research 36, 2959–2966, 2002.
[14] Hussain MA, “Anticholinesterase Properties of Methamidophos and
Acephate in Insects and Mammals,” Bull Environ Contam. Toxicol. 38, 131–138,
1987.
[15] Joshi SC, Sharma P, “Effect of Acephate on Testicular Functions of Albino
Rats,” RJPBCS 4, 137-146, 2012.
[16] Kibong K, Olga GT, David AA, David GC “Destruction and Detection of
Chemical Warfare Agents,” Chem. Rev. 111, 5345-5380, 2011.
[17] Maul JD, Farris JL, “Monitoring Exposure of Passerines to Acephate, Dicroto- phos, and Malathion Using Cholinesterase Reactivation,” Bull. Environ. Cont.
& Toxicol. 73, 682-687, 2004.
[18] Md. Mahajna, Quistad GB, Casida JE, “Acephate Insecticide Toxicity: Safety Conferred by Inhibition of the Bioactivating Carboxyamidase by the Metabo- lite Methamidophos,” Chem. Res. Toxicol 10, 64–69, 1997.
[19] Md. Wasim A, Dwaipayan S, Chowdhury A, “Impact of Pesticides use in
Agriculture: Their Benefits and Hazards,” Interdiscip Toxicol. 2, 1–12, 2009.
[20] Miyazaki A, Nakamura T, Kawaradani M, Marumo S, “Resolution and Bio- logical Activity of both Enantiomers of Methamidophos and Acephate,” J. Agric. Food Chem. 36, 835–837, 1988.
[21] Mohiyuddin SS, Reddy SR, Kumar LA, Doss PJ, “Acephate Induced Altera-
tions in Mg2+ ATPase and Na+-K+ ATPases of Different Brain Regions of
Albino Rats,” The Bioscan. 5, 153–156, 2010.
[22] Nisar K, Kumar J, Shakil NA, Walia S, Parmar BS, “Controlled Release For- mulations of Acephate: Water and Soil Release Kinetics,” J Environ Sci Health B. 44, 533-537, 2009.
[23] Pinjari AB, Novikov B, Rezenom YH, Russell DH, Wales ME, “Mineraliza- tion of Acephate, a Recalcitrant Organophosphate Insecticide Is Initiated by a Pseudomonad in Environmental Samples,” PLoSONE 7, 31963-31970, 2012.
[24] Poovala VS, Kanji VK, Tachikawa H, Salahudeen AK, “Role of Oxidant Stress and Antioxidant Protection in Acephate- Induced Renal Tubular Cytotoxici-
ty,” Toxicol. Sci. 46, 403-409, 1998.
[25] Rattner BA, Hoffman DJ, “Comparative Toxicity of Acephate in Laborato- ryMice, White-footed mice, and Meadow voles, Arch,” Environ. Contam. Toxi- col. 13, 483-491, 1984.
[26] Raymond A, Cloyd AA, Clifford SS, “Effects of Spinosad and Acephate on
Western Flower Thrips Inside and Outside a Greenhouse,” HorTech. 10, 350-
362, 2000.
[27] Robina F, Shaukat SF, Abida KK, Umar F, “Ultrasonic Induced Decomposi- tion of Methidathion Pesticide,” J. Appl. Sci. 8, 140-143, 2008.
[28] Singh AK, “Kinetic Analysis of Acetylcholinesterase Inhibition by Combina- tions of Acephate and Methamidaphos,” Toxicol. 42, 143–156, 1986.
[29] Swapnil S, Phugare YB, Gaikwad JPJ, “Biodegradation of Acephate using a Developed Bacterial Consortium and Toxicological Analysis using Earth- worms (Lumbricus terrestris) as a model animal,” Int. Biodet. Biodeg. 69, 1–9,
2012.
[30] Szeto SY, MacCarthy HR, Oloff PC, Shepherd RF, “The Fate of Acephate and
Carbaryl in Water,” J. Environ. Sci. Health B. 14, 635–654, 1979.
[31] Wang L, Wen Y, Guo X, Wang G, Li S, “Degradation of Methamidophos by Hyphomicrobium species MAP-1 and the Biochemical Degradation Pathway,” Biodegradat. 21, 513–523, 2010.
[32] Wang L, Yin Q, Zhang M, Wang J, “Solubility of Acephate in Different Sol- vents from (292.90 to 327.60) K,” J. Chem. Eng. Data,” 52, 426–428, 2007.
[33] Weina F, Yunhai W, Chi H, Jinglian H, “Photocatalytic Degradation of Acephate on ZnFe2O4-TiO2 Photocatalyst under Visible-Light Irradiation,” J. Adv. Oxid. Technol. 15, 177-182, 2012.
[34] White T, Spassova D, Jiang Y, Singh AK, “Physicochemical, Molecular-Orbital and Electronic Properties of Acephate and Methamidophos,” Pharmacol. Toxi- col. Endocrinol. 119, 107–111, 1998.
[35] WHO, “Pesticides Residues in Food – Joint FAO/WHO Meeting on Pesti- cides Residues in Food,” 2002.
[36] Yen JH, Lin KH, Wang YS, “Potential of the Insecticides Acephate and
Methamidophos to Contaminate Groundwater,” Ecotoxicol Environ Saf. 45,
79–86, 2000.
IJSER © 2013 http://www.ijser.org





