International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 1
ISSN 2229-5518
Characterization of Mono-crystalline Silicon from Rice Husk Ash
OLAWALE, O*, AKINMOLADUN, A.I1 OYAWALE, F.A.2 and Atiko Rejoice3
Corresponding Author: lamstock2@yahoo.com
*,1 Industrial and Production Engineering Department, University of Ibadan, Nigeria
2 Office of Dean of Student, Samuel Adegboyega University, Ogwa, Edo State, Nigeria
3 Chemistry Department, Gombe State, University, Nigeria.
Abstract — In this research, High purity silica was prepared by reflux raw rice husks (RRH) in oxalic acid which further removed the impurities, then burnt at 650°C for 3 hours. The aim of this research is characterisation of micro crystalline silicon production from rice husk ash. The obtained white ash was examined by XRD. Silicon was prepared by metallothermal reduction of pure silica at 650°C for 3 hours, using magnesium (99% purity) as a reducing agent. The results from X Ray Diffraction (XRD) patterns and Raman spectra indicated that the powder was micro crystalline silicon. The surface morphology of the powder was revealed by Scanning Electron Microscope (SEM) showing porosity due to acid leaching.
Index Terms:, Micro crystalline Silicon, Rice Husk, Rice Husk Ash, Scanning Electron Microscope (SEM), Silica, X-Ray Diffraction (XRD), Raman
Spectra
—————————— • ——————————
1. INTRODUCTION
The fluctuations of availability and feedstock cost determine the profitability of photovoltaic manufacturers, their production volume and expansion plans. The explosive growth of the solar cell industry has already driven up the price of electronic grade silicon and immediate solutions to the feedstock supply crunch is not clear [6]. Rice husk has been found to have a high content of hydrated silica from which silicon can be extracted. Silicon oxide is normally generated from sand that is extracted after a fusion of high temperature. Characterisation temperature was determined via the temperature at which the highest Specific Surface Area (SSA) and highest amount of silica were observed which was 7000C [8].Presently, having no commercial value in itself, Rice Husks (RH) usually ends up being burned in open spaces, thus causing environmental pollution and disposal problems. Due to the need to conserve energy and resources, efforts have been made to burn the husk under controlled conditions and to utilize the resultant ash as building, semiconductor, composite, and abrasive materials [10]. Also, ash is an active catalyst and a good
material for catalyst support because of its high surface area [2].
Rice husks are known to have a relatively high content of inorganic compounds. According to [11], depending on the soil content, some hazardous metal elements may be included in them. Combustion is the conventional technique for rice husk to exploit the calorific value and to obtain silica for commercial use, but cations such as K+, Al3, P+5, Fe3+ and Mn can remain in rice husk ash(RHA) as oxides, decreasing its purity and further limiting its use. Moreover, in the direct combustion process of rice husk, the obtained rice husk ash consists of many black particles, which are very difficult to be fully burnt off. The high impurity of potassium (K) content is generally recognised to be the cause [6].It produces high ash content, varying from 13 to 29wt % depending on the variety, climate and geographical location. The ash is largely composed of silica (87-97%) with small amounts of inorganic salts. Due to its high silica content, rice husk has become a source for preparation of a number of silicon compounds such as silicon carbide, and silicon nitride
[3]. Its silica has fine particle size and high reactivity
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 2
ISSN 2229-5518
silica and is used to produce solar grade silicon. Nano- crystalline silicon (nc-Si), sometimes also known as microcrystalline silicon (J-c-Si), is a form of porous silicon. It is an allotropic form of silicon with para- crystalline structure: which is similar to amorphous silicon (a-Si), in that it has an amorphous phase. Where they differ, however, is that nc-Si has small grains of crystalline silicon within the amorphous phase. This is in contrast to polycrystalline silicon (poly-Si) which consists solely of crystalline silicon grains, separated by grain boundaries. The difference comes solely from the grain size of the crystalline grains. Most materials with grains in the micrometer range are actually fine-grained poly-silicon, so nano-crystalline silicon is a better term. It has many useful advantages over a-Si, one being that if grown properly it can have higher electron mobility, due to the presence of the silicon crystallites. It also shows increased absorption in the red and infrared wavelengths, which make it an important material for use in a-Si solar cells. Its important advantage however, is that it has increased stability over amorphous-silicon, one of the reasons being because of its lower hydrogen concentration [9]. Demand and capacity of silicon production in tons is as shown in Figure 1.
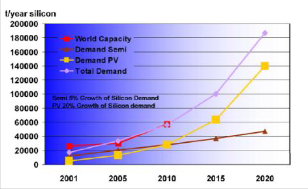
Fig.1: Demand and capacity of silicon production in tons
Source: [7].
Micro crystalline silicon production is an attractive research for solar cell material application. The emphasis of this paper is to characterise the characterisation of micro crystalline silicon production
from rice husk ash.
2. MATERIALS and METHODS
2.1Purification of the Rice Husks
Raw Rice husks (RRH) collected from Osun State, Nigeria was thoroughly washed at least 4 times using distilled water and dried in an oven. The RRH was further refluxed with oxalic acid which removes the impurities present further. It was dried in the oven and used for further silica preparation. RRH was subjected to calcination at 7000C heating rate of 10 oC/min and hold for 3h.
2.2 Preparation of High Purity Silica
The acid-treated RRH surface structure and surface morphology of silica was analysed via XRD and SEM.
2.3 Silicon Characterization
Metallothermal reduction process was employed for the preparation of silicon. Silica produced and Mg powders were homogeneously ground to obtained fine powder using agate mortar. The mixture was pyrolyzed in a tube furnace at 650°C for 3hour.A portion of the obtained brownish powder (as -reduced silica) was analysed by XRD and subsequently washed with HCl: H2O and HF: H2O.This is the leaching procedure adapted from [1] and [4]. The analysis of silicon was performed using XRD and Raman Spectra.
3. RESULTS and DISCUSSION
Fig.2 (a) shows the XRD of the observed silica, Fig. 2(b) and 2(c) show images taken by XRD and scanning electron microscope (SEM) of the silicon extracted from the Rice Husk Ash (RHA). SEM shows agglomerated particles vary in sizes confirming the tendency to cluster.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 3
ISSN 2229-5518
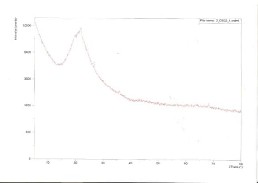
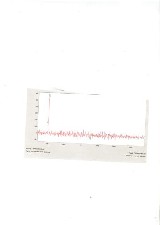
Fig 2(a) XRD result of the observed Silica
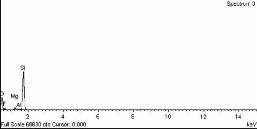
Fig.2 (b ) XRD of Silicon Produced
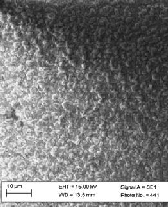
Fig 2 (c ): SEM of Silicon Produced
Raman peak of prepared sample at 650°Cfor 3 hours as shown in Figure 3, which materialise at the wave number of 520cm-1while for standard silicon it is at
519.728 cm-1. This shows that micro crystalline silicon
was produced from the Raman Spectra result observed.
Fig 3: Raman Spectra of Silicon produce
4. CONCLUSION
High purity silica (SiO2) was prepared by reflux RRH in oxalic acid, and then pyrolyzed at 650°C for 3 hours. This silica was further used as a starting material for silicon (Si) preparation by metallothermal reduction process, using Mg as a reducing agent at 650°C for 3 hours.. The obtained Si was analysed by XRD and confirmed by Raman spectra to be micro crystalline silicon. The prepared micro crystalline silicon was compared with the standard silicon.
5. REFERENCES
[1.]Amick, J.A. 1982. Purification of rice hulls as a source of solar grade silicon for solar cells.J. Electrochem. Soc.
129(4): 864 – 866
[2.] Ezzat Rafiee1*, Shabnam Shahebrahimi1, Mostafa Feyzi1 and Mahdi Shaterzadeh2 (2012) Optimization of synthesis and characterization of nanosilica produced from rice husk (a common waste material) International Nano Letters, 2:29. doi:10.1186/2228-5326-2-29
[3.] Hanna, S. B.; Farag, L. M. and Mansour, N. A. L.(1984) Pyrolysis and Combustion of Treated and Untreated Rice Hulls. Thermochimica Acta. Vol: 81:
Pp:77-86.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research Volume 4, Issue 2, February-2013 4
ISSN 2229-5518
[4.] Hunt, L.P.,Diskmukes, J.P. and Amick, J.A. (1984).Rice hulls as a raw material for producing silicon. J. Electrochem. Soc. 7: 1683 – 1686.
[5.] Istratov, A.A.; Buonassisi, T.,Pickett, M.D.; Heuer, M and Weber, E.R (2006) Control of metal impurities in “dirty” multicrystalline silicon for solar cells. Journal of Material Science and Engineering. Issue:B Vol:134 Pp.:
282–286
[6.] Krishnarao,R. V.,Subrahmanyam, J. and Jagadish Kumar,T.(2001)’Studies on the formation of block particles in rice husk silica ash’. Journal of European Ceramic Society.Vol:21, Pp:99-104
[7.] Müller A., Ghosha M., Sonnenschein R. and Woditsch P(2006) Silicon for photovoltaic applications. Materials Science and Engineering B, 134 257-262
[8.] Olawale, O. and Oyawale, F.A. (2012) Characterization of Rice Husk via Atomic Absorption Spectrophotometer for Optimal Silica Production.Pp:210-213. ISSN 2224-3577
[9.]Www.Wiki.Nano crystalline silicon (2011) April,2011. Retrieved on 20th November, 2011
[10.] Siqueira, E.J, Yoshida, IVP, Pardini, L.C, Schiavon, M.A (2009) Preparation and characterization of ceramic composites derived from rice husk ash and polysiloxane. Ceram. Int. 35, 213
[11.]Umedo Junko, Kondoh Katsuyoshi and Muchiura Yoshisada(2008).Environmentally benign reuse of agricultural wastes to prepare high purity silica from rice husks.TransactionsofJWRI,Vol:37,No:2,Pp:17-
21,Ibafaki,Osaka, 567-0047,Japan
IJSER © 2013 http://www.ijser.org