International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 790
ISSN 2229-5518
Breast Cancer: CD3+ Tumor Infiltrating Lymphocytes Differences among Molecular Subtypes
Nidhal A. Mohammed, Sahera A. Ali, Saad M. Saleh, Ahmed S. Abood
Abstract--CD3 is a receptor present on all T lymphocytes. Controversy still surrounds the prognostic role of tumor infiltrating lymphocytes (TILs) within a tumor microenvironment. The present study was done to evaluate CD3 TIL in BC patients and correlation with the molecular subtypes. Sixty-one cases as patients group and seven have been selected as normal group for the study. IHC was used to evaluate the expression of ER, PR, Her2\neu and CD3. Modified Allred scoring was used to evaluate the ER and PR, while Dako Her2 guideline was used for Her2. CD3 assessed by H-SCORE system in two locations (intratumoral and stromal). CD3 shows significant differences in intratumoral lymphocytes and stromal lymphocytes scores among molecular subtypes (p =.047 and .045 respectively). The tumor microenvironment differ according to molecular subtype, moreover, this difference in the microenvironment can modulate the immune response inasmuch CD3+ TIL percentage varies from one molecular subtype to another.
Keywords: Breast cancer, CD3, Molecular types, Hormone receptors, Tumor infiltrative lymphocytes.
1. INTRODUCTION:
—————————— ——————————
2. MATERIALS & METHODS:
N Iraq, breast cancer (BC) is the commonest type of malignancy in females and there is a general trend towards an increase in the frequency of breast cancer as well as increase incidence in younger age group. Patients under 30 years old age formed about 5% of cases, whereas about 75% of the cases occurred in women older than 40 years. The highest number of cases is between 40-50 years old age groups (1). As BC is a genetically and clinically heterogeneous disease (2), the BC classification systems have been developed in order to organize this heterogeneity and
standardize the language.
In addition, BC is immunogenic, the immune system can
play a dual role in breast cancer, both promoting
tumorigenesis through inflammatory pathways that also suppress adaptive immunity and preventing tumor formation through active immune surveillance (3).
CD3 antigen is a receptor glycoprotein present on all T
lymphocytes. Controversy still surrounds the prognostic role
of tumor infiltrating lymphocytes (TILs) within a tumor
microenvironment. While higher concentration of CD3 TIL has been shown to link with favorable outcome in oropharyngeal cancer (4) , a low CD3 count has been reported to predict a shorter disease free survival in colon and cervical cancer (5),(6).
The present study was done to evaluate the density,
localization and distribution of CD3 TIL in BC patients. The findings were correlated with the molecular subtypes.
————————————————
• Nidhal A. Mohammed, College of Biotechnology, Al-Nahrain University, Iraq E-mail: dr.nidhalmohammed@yahoo.com
• Sahera A. Ali, College of Medicine, Baghdad University, Iraq.
• Saad M. Saleh, College of Education, Al-Iraqia University, Iraq.
• Ahmed S. Abood, College of Education, Al-Iraqia University, Iraq.
Ninety-five of fresh samples and paraffin embedded tissue blocks from female patients with breast mass, during the period between May 2012 till February 2013. Their age ranged from (16 to 70) years. Thirty control samples were taken from normal breast tissue (dead females) in Iraqi center of forensic medicine. Pathological data including: histologic tumor type, tumor grade, tumor stage and lymph node status, were revised and confirmed by a specialist histopathologist. Out of the total ninety-five cases, only sixty- one patients group and out of the thirty normal sample, only seven have been selected as normal group for the study. According to clinic-pathological examination (H&E), the patients distributed into Malignant, Benign and Reactive. In order to approximate the molecular subtypes three markers had been used (ER, PR and Her2) (7) as shown in (Table 1).
Table 1: Approximate Molecular Subtype Using Three Markers
Marker | Luminal A | Luminal B | HER2 | Basal-like |
ER | + | + | - | - |
PR | + | + | - | - |
Her2 | - | + | + | - |
Immunohistochemistry was used to evaluate the expression of ER, PR, Her2\neu and CD3. All of monoclonal Abs, staining kits, Abs diluent, Ag retrieval solution, Mayer’s hematoxylin and mounting medium used in this study were produced by DakoCytomation, Denmark. The staining procedures were performed according to manufacturer’s instructions. Modified Allred score system (8) was used to evaluate the immune-expression of the ER and PR, while Dako Her2 guideline was used for Her2. In order to evaluate
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 791
ISSN 2229-5518
the CD3 immunostaining, the semi-quantitative analysis (H- SCORE system),as in (1) was used to assess percentages and staining intensity of the cells stained (9). The H-SCORE was calculated using the following equation:
𝑯 − 𝒔𝒔𝒔𝒔𝒔 = ∑ 𝑷𝑷 (𝑷) (𝐢 = 𝟎, 𝟏, 𝟐, 𝟑, 𝐏𝐢 = 𝟎, 𝟏𝟎𝟎%) (1)
{(i) means the intensity of staining, i.e. no staining = 0, weak
staining = 1, moderate staining = 2 and strong staining = 3. Pi represents percentages of stained cells with intensities varying from 0 to 100}.
Therefore, the H-SCORE ranges from 0 to 300, H-SCORE>0 is considered as positive staining and H-SCORE = 0 is considered as a complete negative staining (10). Two location were scored in each case section, intratumoral and stromal, and two high power field for each location.
Nuclear staining of the cells for both ER and PR was evaluated using modified Allred scoring guideline. According to Dako ER & PR interpretation manual, a positive is defined as TS≥ 3. Membrane staining of the cells was evaluated using the DAKO/HER2 scoring system. Only score +3 has considered a positive and the other scores as negative (11)(11).
According to ER, PR and Her2 results, patients group were classified as luminal-A (ER and/or PR positive, HER2-), luminal-B (ER and/or PR positive, HER2+), HER2-Rich (ER-,
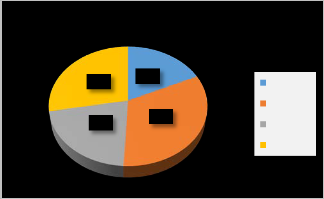
Table 2: Estrogen receptor positivity in patients group. Table 3: Progesterone receptor positivity in patients group. Table 4: HER2 positivity in patients group.
Molecular Subtypes
PR-, HER2+), and basal (ER-, PR-, HER2-) (11)(12).
In brief, intratumoral T-lymphocytes were defined as T- lymphocytes located within tumor cell nests or in direct contact with the breast cancer malignant epithelial cells, whereas stromal T-lymphocytes were defined as T- lymphocytes in the stroma without direct contact with the cancer cells (13)(13).
28%
21%
18%
33%
BasalLike Her2Rich LuminalA LuminalB
The data were statistically analyzed depending on the nature of the character, according to Snedecor and Cochran (1981) and data processing was done by using Statistical Package of Social Science (SPSS) version 22. Data description was presented as means with their standard errors (SE) and standard deviation (SD) were calculated to reflect the size and precision of the estimated values. The independent sample t-test of significance was used for the comparison between two groups. ANOVA test was used to find the differences among three groups or more. The lowest level of significance chosen to be when the probability (p) was less than or equal to 0.05 (p≤0.05).
3. RESULTS:
According to the results of ER, PR and HER2 (See Tables 2, 3,
4) the patients group divided into the molecular subtypes.
The most frequent molecular subtype is Her2-rich (33%);
second group is Luminal B (28%).followed by Luminal A
(21%) and Basal-like (18%). See figure (1).
Figure 1: Molecular subtypes frequency in patient group.
With use of independent sample T-test, immunostaining of CD3 show high significant differences between patients and normal groups in both tumor nest and stromal score (p ≤
0.001 and 0.002 respectively) See Table (5). Moreover,
Immunostaining of CD3 shows high significant differences
(p ≤ 0.001) between intratumoral lymphocytes and stromal
lymphocytes scores in patients group (See Table 6).
Immunostaining of CD3 shows significant differences in intratumoral lymphocytes and stromal lymphocytes scores among molecular subtypes (p = .047 and .045 respectively) See Table (7).
Table 5: Independent sample T-test for CD3 expression in normal and patients group
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 792
ISSN 2229-5518
than Alwan’s (721). Our results of HER2 positivity come in agreement with that of Al-Khafaji (15)in which he stated that there is increase in HER2 expression. On the other hand, HER2 positivity percentage in this study is more than that of Alwan’s (14)(14). The differences in the detection results might be due to the same reason previously mentioned.
Table 6: Descriptive data for CD3 expression differences in patients group.
CD3 | Range | Min. | Max. | Mean | S.D. | P value |
CD3 | Range | Min. | Max. | Stat. | S.E. | S.D. | P value |
Tumor | 300 | 0 | 300 | 108.33 | 8.957 | 63.966 | ≤ 0.001 |
Stroma | 300 | 0 | 300 | 102.45 | 10.006 | 71.459 | ≤ 0.001 |
Table 7: Descriptive data and ANOVA for CD3 expression within molecular subtypes.
CD3 | Mean | Std. Dev. | Std. Error | 95% Confidence Interval for Mean | Min. | Max. | P value |
CD3 | Mean | Std. Dev. | Std. Error | Lower Bound | Upper Bound | Min. | Max. | P value |
Tumor | Luminal- A | 141.67 | 76.376 | 22.048 | 93.14 | 190.19 | 50 | 300 | .047 |
Tumor | Luminal- B | 95.00 | 49.721 | 15.723 | 59.43 | 130.57 | 0 | 200 | .047 |
Tumor | Basal- Like | 127.27 | 60.678 | 18.295 | 86.51 | 168.04 | 50 | 200 | .047 |
Tumor | Her2- Rich | 81.94 | 54.101 | 12.752 | 55.04 | 108.85 | 25 | 200 | .047 |
Tumor | Total | 108.33 | 63.966 | 8.957 | 90.34 | 126.32 | 0 | 300 | .047 |
Stroma | Luminal- A | 141.67 | 76.376 | 22.048 | 93.14 | 190.19 | 50 | 300 | .045 |
Stroma | Luminal- B | 95.00 | 49.721 | 15.723 | 59.43 | 130.57 | 0 | 200 | .045 |
Stroma | Basal- Like | 118.18 | 75.076 | 22.636 | 67.75 | 168.62 | 0 | 200 | .045 |
Stroma | Her2- Rich | 70.83 | 65.445 | 15.426 | 38.29 | 103.38 | 0 | 200 | .045 |
Stroma | Total | 102.45 | 71.459 | 10.006 | 82.35 | 122.55 | 0 | 300 | .045 |
4. DISCUSSION:
Our findings of ER and PR positivity (27.9 % and 29.5%) are lower than the results of local study conducted by Alwan (14). Alwen’s reported that ER and PR positivity were demonstrated in (65.1%) and (45.1%) respectively of the studied group. The difference between our findings and the previously mentioned report is due to the studied population size variation, our patients group is smaller (61)
According to hormones receptors our findings for the molecular subtypes were Her2-rich (33%); second group is Luminal B (28%).followed by Luminal A (21%) and Basal- like (18%). Such as these results are in discordancy with what Malhotra et al., had reviewed. Whereas, they reviewed several reports and stated different frequencies percentages for the molecular subtypes; Luminal A (40%), Luminal B (20%), Basal-like (15-20%) and Her2-rich (10-15%) (16). The explanation for current situation is that our findings yielded from the use of IHC technique and for three markers only, while the reviewed reports by Malhotra et al.,(16) had use more sophisticated techniques like PCR and Microarray and for more than three marker. In addition, the ethnic variation between the studied cohorts may play role in the resulted frequencies. Shawarby et al., had reviewed that there are striking differences in compared prevalence patterns in the western and other regionally based studies (17).
the result of high significant differences in the CD3 immuno-expression between the intratumoral and stromal areas for patients group come (p ≤ 0.001), in some extent, in concordance with the results of Rathore et al., (18)(18)in which the studied group consist of triple-negative BC cases. Explanation for that differences is that might be part of the immune response where the effectors cells activated and accumulated in the site of foreign Ags (19),(20). Beside to the effect of tumor micro-environment which can be modulator to immunological effector cells (21)(22),(23),(24).
Depending upon the molecular subtyping of the patients group the immunostaining for CD3 shows significant differences in both intratumoral and stromal scores among the molecular subtypes (p=.047 and .045 respectively). These findings are consistent with many studies investigate the association between CD3+ tumor infiltrative lymphocytes and certain clinicpathological parameters in regard with the hormones receptors. Rathore et al.,(18)had conclude an association between high CD3+ TIL both intratumoral and stromal with survival rate in Basal-Like BC (triple negative BC). Bedri et al., (25)study shows that intratumoral CD3+ TIL were significantly higher in ER/PR negative Her2/neu positive tumors (i.e. HER2-Rich molecular subtype) compared to triple negative breast cancers (i.e. Basal-Like). Calabr`o et al., (26)(26)had stated a poorer overall survival in ER+ patients and better overall survival in ER−patient in association with TIL. Rody et al., (27)reported that there is association between intratumoral and stromal CD3+ TIL with better recurrence free survival in cases who had HER-2+. Our results, with the
IJSER © 2014 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 793
ISSN 2229-5518
support of the previously mentioned studies, suggest that the tumor microenvironment differ according to molecular subtype, moreover, this difference in the microenvironment can modulate the immune response inasmuch CD3+ TIL percentage varies from one molecular subtype to another.
REFERENCES:
[1] Iraqi Cancer Board I. C. R. C. (2011). Results of Iraqi National Cancer Registry, (1975-1978; 1989-1991; 1995-1997, 1998-2000,…2009-2011). Ministry of Health. Baghdad- Iraq.
[2] Stingl J and Caldas C (2007). Molecular heterogeneity of breast
carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer;
7:791-9.
[3] Emens LA (2012). Breast cancer immunobiology driving
immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. ; 12(12): 1597–1611. doi:10.1586/era.12.147.
[4] Rajjoub S, Basha SR, Einhorn E, Cohen MC, Marvel DM, Sewell DA
(2007). Prognostic significance of tumor-infiltrating lymphocytes in oropharyngeal cancer. Ear Nose Throat J;86(8):506-11.
[5] Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent
DJ (2009). Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology;137(4):1270-9.
[6] Ancuta E, Ancuţa C, Zugun-Eloae F, Iordache C, Chirieac
R,Carasevici E (2009). Predictive value of cellular immune response in cervical cancer. Rom J Morphol Embryol;50(4):651-5.
[7] Brenton JD, Carey LA, Ahmed AA, Caldas C (2005).Molecular
Classification and Molecular Forecasting of Breast Cancer: Ready for Clinical Application?. . Journal of Clinical Oncology;23(29). by American Society of Clinical Oncology 0732-183X/05/2329-
7350/$20.00.
[8] Allred DC, Harvey JM, berardo M, Clark GM (1998). Prognostic and
predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol.,11:155-68.
[9] Lee HJ, Choe G, Jheon S, Sung SW, Lee CT, Chung JH (2010). CD24, a novel cancer biomarker, predicting disease-free survival of non- small cell lung carcinomas: a retrospective study of prognostic factor analysis from the viewpoint of forthcoming (seventh) new TNM classification. J Thorac Oncol. 5(5):649-57. doi:
10.1097/JTO.0b013e3181d5e554.
[10] Jiang J, Jin M-S, Kong F, Cao D, Ma H-X, Jia Z, Wang YP, Suo J, Cao
X (2013). Decreased Galectin-9 and Increased Tim-3 Expression Are Related to Poor Prognosis in Gastric Cancer. PLoS ONE 8(12): e81799. doi:10.1371/journal.pone.0081799
[11] Vermeulen JF, van Brussel ASA, van der Groep P, Morsink FHM,
Bult P, van der Wall E, van Diest PJ (2012).Immunophenotyping
invasive breast cancer: paving the road for molecular imaging. BMC Cancer, 12:240.
[12] Kornegoor R, Verschuur-Maes AH, Buerger H, Hogenes MC, de Bruin PC, Oudejans JJ, van der Groep P, Hinrichs B, van Diest PJ (2012).Molecular subtyping of male breast cancer by immunohistochemistry. Mod Pathol., 25:398–404.
[13] Chen Z, Chen X, Zhou E, Chen G, Qian K, Wu X, Miao X, Tang Z (2014). Intratumoral CD8+ Cytotoxic Lymphocyte Is a Favorable Prognostic Marker in Node-Negative Breast Cancer. PLoS ONE
9(4): e95475. doi:10.1371/journal.pone.0095475.
[14] Alwan N (2010). Breast cancer: demographic characteristics and
clinico-pathological presentation of patients in Iraq. EMHJ. 16(11). [15] AL-Khafaji QA (2010). Molecular Assessment of BRCA 1, P53 and
KRAS Oncogenes by Multiplex PCR and HER-2 Detection Using Electrochemical Biosensor in Iraqi Breast Cancer Women. A thesis submitted to the college of medicine and committee of graduate studies of AL-Nahrain University, for PhD in Microbiology.
[16] Malhotra GK, Zhao X, Band H, Band V (2010). Histological,
molecular and functional subtypes of breast cancers. Cancer Biology
& Therapy 10:10, 955-960.Landes Bioscience.
[17] Shawarby MA, Al-Tamimi DM, Ahmed A (2013). Molecular Classification of Breast Cancer: An Overview with Emphasis on Ethnic Variations and Future Perspectives. Saudi Journal of Medicine & Medical Sciences. 1(1):14-19.
[18] Rathore AA, Goel MM, Makker A, Kumar S, Srivastava A (2013).
Prognostic Impact of CD3 Tumor Infiltrating Lymphocytes in
Triple-negative Breast Cancer. Ind J. of Cl. Practice.24 (4).
[19] Owen JA, Punt J, Stranford SA (2013). KUBY Immunology.7th
Ed.Ch19. ISBN-10: 1-4641-3784-6. by W. H. Freeman and Company.
[20] Whiteside TL, Robinson BWS, June CH, Lotze MT (2013). Principles
of tumor immunology.in Rich RR et al., Clinical immunology:
principles and practice.4th Ed.Ch 76.Pgs 925-935. ISBN: 978-0-7234-
3710-9. Elsevier.
[21] Cruz-Merino LD, Barco-Sánchez A, Carrasco F.H., et al., (2013). New
Insights into the Role of the Immune Microenvironment in Breast
Carcinoma. Clin and Develop Immuno. Volume 2013, Art ID
785317. Hindawi Publishing Corporation.
[22] Witz IP and Levy-Nissenbaum O (2006). The tumor microenvironment in the post PAGET era. Cancer Lett 242:1–10.
[23] Witz IP (2008). Tumor-microenvironment interactions: dangerous liaisons. Adv Cancer Res 100:203–229.
[24] Weinberg RA (2008). Coevolution in the tumor microenvironment.
Nat Genet 40:494–495.
[25] Bedri S, Mohamed M, Sarwath H, Sastry K (2014). Characterization
and quantification of tumor infiltrating lymphocytes in breast cancer. Journal for ImmunoTherapy of Cancer 2(Suppl 1):P9.
[26] Calabr`o A, Beissbarth T, Kuner R, et al., (2009). Effects of infiltrating lymphocytes and estrogen receptor on gene expression and prognosis in breast cancer. Breast Cancer Research and Treatment.
116(1):.69–77.
[27] Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, et al., (2009). T-
cell metagene predicts a favorable prognosis in estrogen receptor- negative and HER2-positive breast cancers. Breast Cancer Research.
11(2); article R15.
IJSER © 2014 http://www.ijser.org
