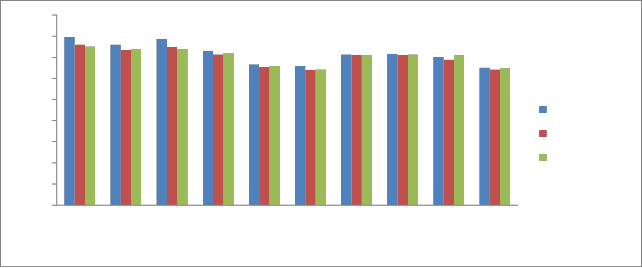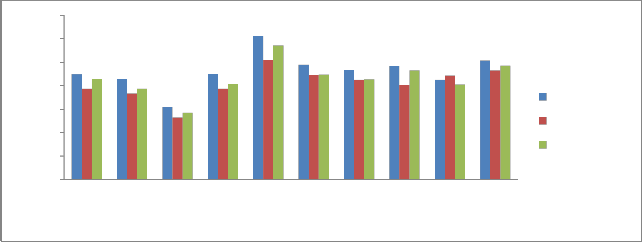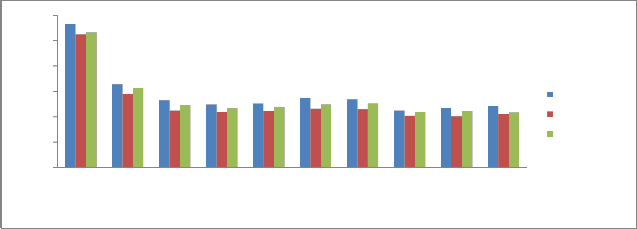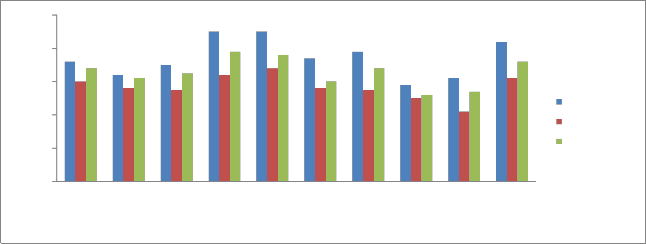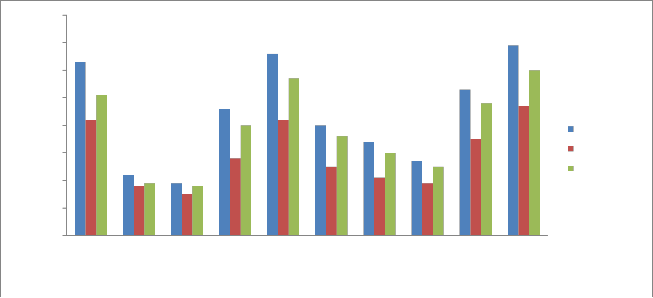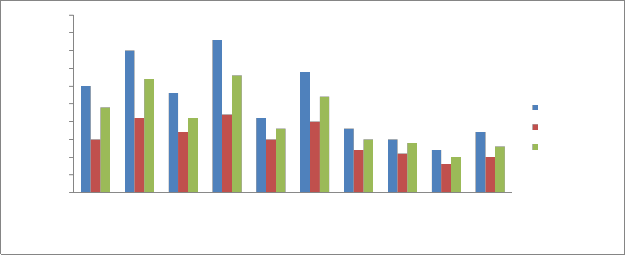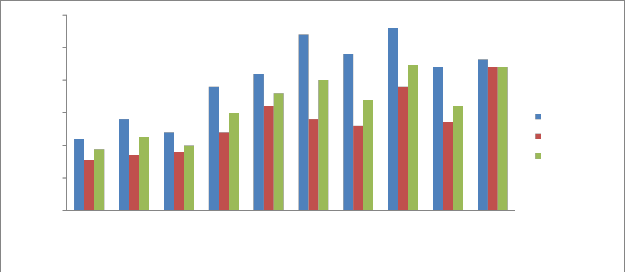International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 787
ISSN 2229-5518
Assessment of impacts by Industries on sediments of Kabini river around Nanjangud Industrial area, Karnataka ,India
Vivek Krishnanandan and Srikantaswamy S
Abstract:
Degradation of aquatic environment is one of the major problems caused by population explosion and industrialization. It has been observed
that aquatic sediments absorb toxic chemicals to many times higher than water column concentration, so determination of water quality alone cannot give a clear idea about the extent of pollution. In order to determine the physico-chemical characteristics and heavy metals concentrations in sediments of Kabini river a baseline study was conducted. Ten sampling stations were selected across the study area of Kabini River flowing through industrial area of Nanjangud. The studies were carried out for three seasons to determine the seasonal variations in physico-chemical characteristics of sediments. pH of the sediments were in the range of 6.38-7.96, EC 9.18 dS/cm -30.60 dS/cm, calcium ranged from 2 mg/kg - 5.7mg/kg, magnesium
0.29 mg/kg -1.93 mg/kg, sodium and potassium were in the range of 7.2 mg/kg - 11.1mg/kg, 2.1 mg/kg - 3.4mg/kg respectively and presence of
significant amounts of nutrients like phosphates, nitrates and sulphates showed river is becoming r ich in nutrients. All the parameters showed considerably less concentrations during monsoon season. The present study indicates that there is increase in the amounts of nutrients in the sediments of Kabini River due to discharge of effluents and sewage dir ectly to the river. The heavy metal study across the area showed the presence of Iron, Copper, Chromium, Lead, Zinc and Nickel. Only copper was found to be above the standards set by USEPA and it showed that sediments are heavily polluted by copper.
Key words: Kabini River, Sediments, Nutrients, Effluent, Heavy metals.
Introduction:
Sediment is defined as particles derived from soil or rock that have been or being transported by water or wind. It can come from
soil erosion or from the decomposition of plants and animals. Sediment is the loose sand, silt and other soil particles that s ettle at the bottom of a body of water [43]. W ind, water and ice help to carry these particles to rivers, lakes and streams. Sediment strata serve as an important habitat for the benthic macro invertebrates whose metabolic activities contribute to aquatic productivity [1]. Sedimentation is a natural spontaneous process and its analysis has great significance in water quality study. Sediments act as both carriers and sinks for contaminations in aquatic environment. These are integral part of aquatic ecosystem which acquires properties due to exchange of chemical parameters of different water bodies. Sediments also play an important role in determining the morphology of water system. Human induced modifications of vegetative covers in river basins may cause strong geomorphic responses by disturbing sediment supply, transport and deposition regimes. Sediment pollution is a major problem around the world because sediments threaten water supplies and recreation and causes harm to fish and plant communities.
Sediments are ecologically important components of the
aquatic habitat, which play a significant role in maintaining the trophic status of any water body [38]. Sediments near urban areas commonly contain high levels of contaminants [17],[25]. which constitute a major environmental problem faced by many anthropogenically impacted aquatic environments [32]. Sediments in rivers do not only play important roles at influencing the pollution, they also record the history of their pollution. Sediments act as both carrier and sources of contaminants in aquatic environment [41].
Corresponding Author: Vivek Krishnanandan, Research Scholar,DOS in Environmental Science, Manasagangotri,Mysore E-mail:vivek.ensci@gmail.com
Co-Author: Srikantaswamy.S, Associate Professor, DOS in Environmental Science, Manasagangotri,Mysore
Geochemical cycle of elements is receiving wide attention due to its need for understanding the pathways of pollutants through our present environment. River processes form a major link in geochemical cycle. Several attempts have recently been made to understand river transport materials. The most attempts are mostly based on study of few low sediment rivers. Various devastating ecological effects and human disasters in the last 40 years have arisen majorly from industrial wastes causing environmental degradation [5]. The discharges from these industries constitute biohazard to man and other living organisms in the environment because they contain toxic substances detrimental to health [7],[8,[13]]. There is alarming and worrisome increase in organic pollutants [31]. Since many effluents are not treated properly, these products are discharged on the ground or in the water bodies [33], and most of these discharges to water bodies accumulate in the system through food chain [33].
The structure of the sediments in the intertribal zone plays a major role in the distribution of the organisms that live in or on them [4]. Sediment input may impact stream communities through a variety of direct and indirect processes including reduced light penetration, smothering, habitat reduction and introduction of absorbed pollutants like pesticides, metals, nutrients. The Physico-chemical parameters of the sediments such as salinity, pH, and organic carbon could also influence the occurrence and abundance of species distributed in them.
The pollution of aquatic ecosystems by heavy metals has assumed serious proportions due to their toxicity and accumulative behavior. River dams are especially at risk of contamination by different contaminants from anthropogenic sources including heavy metals since change of the sediment regime often occurs.
River sediments, as basic components of our environment,
provide foodstuffs for living organisms. They also serve as a sink and reservoir for a variety of environmental contaminants. It has be en recognized that aquatic sediments absorb persistent and toxic chemicals to levels many times higher than the water column concentration [9],[22],[30]]. Namely, when released into the aquatic environment, many anthropogenic chemicals bind or adsorb onto particulate matter. Depending on the river morphology and hydrological conditions, suspended particles with associated contaminants can settle along the watercourse and become part of the bottom sediments, often for many kilometers downstream from the chemical
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 788
ISSN 2229-5518
sources. Trace metals derived from natural inputs and anthropogenic emissions are ubiquitous in the global environment. Consequently, sediment-associated pollutants can influence the concentrations of trace metals in both the water and sediments. In this background, the present study has been attempted to study the physico – chemical parameters and concentrations of heavy metals in the sediments of Kabini River, which in turns gives a clear idea about the pollution status of Kabini downstream.
Materials and Methods:
The present study has been carried out in and around
Nanjanagud, Taluk head quarter of Mysore district, India. Nanjangud is a home to many industries which are mainly located in the Nanjangud Industrial area which is spread across 532 acres (2.15 km2). Najangud Industrial Area is situated near Nanjangud Town, it is about 25 Km
from Mysore and 175 Km. from Bangalore. The area is developed along Mysore – Niligiri National Highway. The Airport is situated at 10
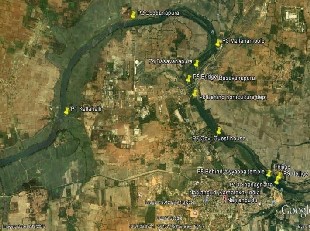
Km. Industrial area situated along the bank of River Kabini (Fig .1). There are 36 major industries, 12 medium industries and 35 small- scale units in Nanjangud. According to National Investigation Agency, Nanjangud is the second highest tax-paying (sales tax of over Rs.400 crore / year) taluk in the State after Bangalore city of Karnataka.
The Kabini River, a confluence of the tributaries from
Panamaram and Mananthavady area originate from W estern Ghats in the W ynad district of Kerala and passes through the Nanjangud industrial area and fl
- - . The source of water of Kabini River is mainly the heavy rainfall from the month of May to October in the W estern Ghats. During this season the rainfall recorded varies from 80 centimeters to 400 centimeters.
guidelines themselves are derived from such data). The sediments were dried at 105°C for 48 h and then ground into a powder.
The sediment (1 g) was weighed into a 100 ml beaker and digested using a nitric/perchloric acid digestion [6,[19]. Concentrated HNO3 (5 ml) was added and the mixture was boiled gently until the volume of liquid was approximately 15 ml. The beaker was cooled and a new portion of 5 ml of HNO3 was added. The sample again was allowed to cool before the addition of 5 ml of trace metal grade HClO4. The samples were heated and the temperature raised gradually to
160°C. The digestion was complete when the white smoke stopped evolving. The concentration of metals in the extracted solution was
determined by air/acetylene flame absorption spectrometry.
Results and Discussion:
The results of physico-chemical analysis are shown in Table1,2 &3 respectively for different seasons and the annual average values of different physico – chemical parameters is shown in Table No.4 .
pH :
pH of the sediments is a measure of their acidity or alkalinity and is one of the stable measurements. Fish, shellfish and aquatic insects have different tolerances to acidic medium and species diversity will decrease along with increased acidification. Young organisms tend to be more sensitive to acidic medium; for example, at a pH of 5, most fish eggs cannot hatch, while only some adult fish will be affected. The toxicity of heavy metals also gets enhanced at particular pH. Acidic sediments also mobilize metals that can be t oxic to aquatic species (e.g., aluminum). In the present study, the pH analysis of sediment samples for the year 2011 was carried out which showed the pH values during pre monsoon varied from 6.5-7.96, during monsoon 6.38 -7.6 and during post monsoon it was in the range
6.42-7.5. The variations in pH during the three seasons are shown in
Fig 2.
During the study period highest pH was recorded at P1 which is due to the presence of carbonates and bicarbonates concentration and the low pH was recorded in P6 which is due to the discharge of industrial effluents. High carbonates and bicarbonates cause calcium and magnesium ions to form insoluble minerals leaving sodium as the dominant ion in sample. The pH of Kabini River sediments was found to be slightly basic in condition.
Figure 1. Study area,Nanjanagud
Sediment sampling were carried out three times in the year
2011 representing different seasons namely Pre-monsoon, Monsoon and Post-monsoon to understand seasonal variations in the physico- chemical properties of the sediments. Sediment sampling was carried out using a dredge sampler.
The samples are separated into particle sizes by wet or dry
6 μ μ B ,
the samples were homogenized and dried (105C) to a constant weight. Homogenization was done by agate pestle and mortar. Physico- Chemical analysis of sediments was carried out according to standard methods [3].
S z “ ”
method [10,[11],[16]. The determination of total content of heavy
metals in sediments is particularly useful to collect information on the genesis of the soil and on the level of contamination. Also, the comparison of sediments to effects-range guidelines involves the use of metal concentrations from total or strong acid digests (because the
Electrical conductivity:
Electrical conductivity (EC) is a measure of ions present in any given sample. The conductivity of a solution increases with the
increase in the amount of ions. The conductivity increases with the increase of ions. Electrical conductivity indicates the presence of soluble salts in the sediments. In the present study, EC of sediment samples shows a range 15.30-30.60 dS/cm during pre monsoon, 13.26
- 25.50 dS/cm in monsoon and for post monsoon it ranges from 14.28-
28.56 dS/cm. EC variations for three seasons are shown in Fig 3.
The EC measurement shows the presence of significant amount of dissolved salts. Highest EC was recorded at P5 which is close to industrial area and also for agricultural fields and it was least in case P9 situated far away from industrial zone.
Sodium (Na)
Sodium is a component of sodium chloride (NaCl), a very important compound found everywhere in the living environment. Sodium is a compound of many foodstuffs for instance of common salt. It is necessary for humans to maintain the balance of the physical fluids system The sodium contents of Kabini river sediments ranges from 8.2 – 11.20 mg/ kg during pre monsoon, 7.2-
9.5 mg/ kg in monsoon and 7.8- 10.6 mg/ kg for post monsoon (Fig 4).
High concentration of sodium was found at P10 and it was less at P8. It is highly soluble in water and there are no precipitating reactions to reduce its concentration. The high value of sodium may be due to increased load of concentrated sewage.
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 789
ISSN 2229-5518
Potassium (K):
Potassium is not an integral part of any major plant
component, but, it plays a key role in a vast array of physiological process vital to plant growth from protein synthesis to maintenance of plant and water balance.
It ranges between 3.1 mg/kg – 4.20 mg/kg during pre monsoon, 2.1 mg/kg -3.4 mg/kg during monsoon and 2.6 mg/kg -3.6 mg/kg during post monsoon (Fig 5). High concentration of available Potassium at station P10 is due to the weathering of minerals and release potassium ions, these ions are adsorbed onto the cation exchange sites. The lower concentration of available potassium at station P9 may be due to less mineral weathering.
Calcium & Magnesium:
Calcium promotes the activity of soil bacteria concerned with the fixation of the free nitrogen or the formation of nitrates from organic forms of nitrogen. Calcium deficiency is commonly associated with the acidity, which will lead to the accumulation of toxic salts of iron, aluminum and manganese in the sediments. Magnesium is essential for all organisms and is not toxic under normal circumstances. Deficiencies of magnesium are much more common than problems concerned with toxicity. Magnesium is a key plant nutrient and is essential for photosynthesis in plants, where it forms the active site in the chlorophyll enzyme molecule.
In the present study, calcium in the sediments ranges from
2.24 mg/kg -5.652 mg/kg during pre monsoon, 2.02 mg/kg -5.241 mg/
kg during monsoon and 2.19 mg/kg - 5.32 mg/ kg during post monsoon
(Figure 6). Magnesium values varied between 0.85 mg/kg – 1.93 mg/
kg during pre monsoon, 0.29 mg/kg – 1.37 mg/ kg during monsoon and
0.73 mg/kg -1.72 mg/ kg during post monsoon of sediment sample (Fig
7). Calcium content is high at P1 and it is least at P8, so also the Magnesium content was lower at station P8 and higher at station P1. Magnesium content in all sampling stations is very close to each other than calcium content. The higher concentration of exchangeable calcium and magnesium is due to the amount of exchangeable forms of calcium & magnesium in sediment samples. It is also attributed to weathering of minerals and their deposition in sediments.
Organic Carbon:
The organic carbon represents the organic matter in the sediment. The dead organic matter gets deposited in the bottom and undergoes chemical and bacterial decomposition. Estimation of organic carbon can serve as an important tool in determining the status of food available to the benthic fauna and also indicates the extent to which the bottom soil is fertile for the subsistence of benthic fauna. The organic carbon also exerts an influence on the available phosphorus level in the soil. The carbon is the common constituent of all organic matter and is a measure of bacterial activity [24].
The percentage of Organic carbon sediments in the study area ranged from 0.19- 0.69 % during pre monsoon, in monsoon 0.18-
0.60 % and 0.15-0.469 % during post monsoon (Fig 8). The results of
organic carbon of study area, shows a highest range of 0.69 % in pre monsoon season at sampling station no. P5 and least amount of at sampling station number P2. The higher content of Organic Carbon in sediments is primarily attributed to the relatively higher supply of organic carbon from the abundant vegetation, agricultural run-off, microbial activity, domestic waste and industrial effluents etc. It is also evident from result of that low organic carbon is due to course sandy nature of the sediments, as the organic carbon variation is largely controlled by the fine fraction of the sediment.
Nitrate (NO3-N):
NO3-N is a necessary primary macro nutrient for plants that stimulates plant growth and is usually added as a fertilizer but can also
be found in sediments as nitrate, ammonia, organic nitrogen or nitrite. Nitrate is a useful form of nitrogen because it is biologically available to
plants and is therefore a valuable fertilizer. However, excessive levels of nitrate in soils and sediments can produce negative health impacts on humans and animals.
The Nitrate content in sediments of the Kabini river ranged from 0.26 mg/kg -4.0 mg/kg during pre monsoon, 0.15 mg/kg -2.20 mg/kg during monsoon and 0.23 mg/kg -2.9 mg/kg during post monsoon (Fig 9). The mean Nitrate content of sediments is 1.004 mg/kg. These values are moderate and are attributed to the medium nutrient level of the Kabini river. Nitrate concentration is not uniformly distributed in all stations .This is attributed to differences in sediment nutrient input from the drainage systems of the various stations.
Phosphate:
Phosphorus is naturally present in water, primarily as
inorganic and organic phosphates. Phosphates can enter aquatic environments in several ways from natural weathering of minerals in the drainage basin, from biological decomposition, or as runoff from human activity in urban and agricultural areas. Phosphorous is imperative in the growth and development of plants and other organisms. Phosphorous is present in water as orthophosphate (PO 3-
), metaphosphate (a phosphate complex) and a limited number of phosphate salts. It is often the limiting factor in production (total biomass produced) and thus is an important nutrient in a water body. While low levels of phosphorus may lead to decreased production in water bodies, high levels have a similarly detrimental effect.
The phosphate content in sediments of the Kabini river ranged from 0.12 mg/kg -0.43 mg/kg during pre monsoon, 0.08 mg/kg -
0.22 mg/kg during monsoon and 0.1 mg/kg -0.33 mg/ kg during post monsoon (Fig 10). The mean Phosphate concentration recorded is
0.205 mg/kg. The phosphate concentration distribution follows the
same pattern as nitrates. The factors which affected nitrate concentration also affect phosphate distribution in the stations. Abowei and Sikoki 2005, reported that, large quantities of phosphate enters rivers and lakes through super-phosphate fertilizer from soil and from chemicals used to improve the performance of detergents, some amount of these gets sinks to the bottom and gets settled in the sediments making the sediment rich in nutrients.
Sulphates:
Sulphates are discharged into the aquatic environment as wastes from industries like pulp and paper mills, textile mills and tanneries. Atmospheric sulphur dioxide (SO2) formed by the combustion of fossil fuels and by the metallurgical roasting process, may also contribute to sulfate content of surface waters. The excess of sulphates will settle down and get accumulated in bottom sediments. These pollutants under some conditions may get released back in to the river water. The presence of sulfate salts in surface water could enhance corrosion of mild steel in the distribution network [26].
The Sulphate content in sediments of the Kabini river ranged from 1.1 mg/kg -2.8 mg/kg during pre monsoon, 0.77 mg/kg -2.20 mg/kg during monsoon and 0.94 mg/kg -2.2 mg/kg during post monsoon (Fig 11). The mean Sulphate concentration obtained in the sediments of Kabini River is 1.65 mg/kg. The sulphate level is considered moderate in the Kabini river sediments. Excess sulphate concentrations in sediments are considered a pollutant. It is important to note that, these pollutants which are accumulated in the sediments will get released into the water under certain conditions. As a result the sulfate and sulfide concentration in water may increase above the permissible limit of 200 mg/L and 2 mg/L respectively, set for inland surface water [42].
Heavy Metals:
The total metal concentrations of heavy metals are tabulated in Table
no.5
Iron:
Iron is one of the essential elements in human nutrition,
however, its presence at elevated concentration in aquatic ecosystems, poses serious pollution and health problems. Toxicity of iron in humans has been found to bring about vomiting, cardiovascular collapse and diarrhea, while iron deficiency may lead to failure of blood clotting.
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 790
ISSN 2229-5518
In the present study the concentration of iron varied from
4157.3- 6118.8 ppm. Highest concentration was recorded at P5 and the lowest was recorded at P1.
Zinc:
Zn plays a biochemical role in the life processes of all
Copper:
Copper is widely used in electrical wiring, roofing, various
aquatic plants and animals; therefore, they are essential in the aquatic
environment in trace amounts. Zinc is used in a number of alloys
including brass and bronze, batteries, fungicides and pigments. Zinc is
alloys, pigments, cooking utensils, piping and in the chemical industry. Copper is present in amunitions, alloys (brass, bronze) and coatings. Copper compounds are used as or in fungicides, algicides, insecticides and wood preservatives and in electroplating, azo dye manufacture, engraving, lithography, petroleum re-fining and pyrotechnics. Copper compounds can be added to fertilizers and animal feeds as a nutrient to sup-port plant and animal growth. Copper compounds are also used as food additives [39]. In addition, copper salts are used in water supply systems to control biological growths in reservoirs and distribution pipes and it forms a number of complexes in natural waters with inorganic and organic ligands [40]. Copper is an essential sub- stance to human life, however, in high concentrations, it can cause anaemia, liver and kidney damage, stomach and intestinal irritation.
In the present study the concentration of copper varied from
57.6- 93.6 ppm. Highest concentration was recorded at P9 and the lowest was recorded at P3. High concentration of copper at P5. Comparing with EPA standards it was found that the sediments are heavily polluted by copper since all the values got are >50ppm at all the stations. This can be attributed to presence of electroplating industries on the banks of river Kabini.
Chromium:
Chromium is one of the bio-chemically active transition metals. W eathering of the earth crust is the primary and natural source
of the chromium in the surface water. Though an essential trace nutrient and a vital component for the glucose tolerance factor, chromium toxicity damages the liver, lungs and causes organ hemorrhages. Chromium compounds are used as pigments, mordents and dyes in the textiles and as the tanning agent in leather. Anthropogenic sources of emission of Cr in the surface waters are from municipal wastes, laundry chemicals, paints, leather, road run off due to tyre wear, corrosion of bushings, brake wires, radiators. According to W HO guideline value for sediment, the concentration of
μ / ,
In the present study the concentration of chromium varied from 6.8-26.1 ppm well within the permissible limits. Highest
concentration was recorded at P5 which is slightly above the established standards of EPA and can be attributed to mixing of effluents from electroplating industries nearby, and the lowest was recorded at P2. All the other concentrations can be attributed to natural background levels. Therefore, chromium poses no threat to the surrounding environment.
Lead:
Lead is a naturally-occurring chemical, it is rarely found in its
I E ’ mineral galena (PbS), and to a lesser extent as anglesite (PbSO4) and cerussite (PbCO3) Lead minerals are found in association with zinc, copper, and iron sulfides as well as gold, silver, bismuth, and antimony minerals. Lead released from natural sources, such as volcanoes, windblown dust, and erosion, are minor compared with anthropogenic sources. Industrial sources of lead can result from the mining and smelting of lead ores, as well as other ores in which lead is a by- product or contaminant. In these processes, lead may be released to land, water, and air. Electrical utilities emit lead in flue gas from the burning of fuels, such as coal, in which lead is a contaminant. Because of the large quantities of fuel burnt by these facilities, large amounts of lead can be released.
In the present study the concentration of Lead varied from
5.1-14.6 ppm. Highest concentration was recorded at P5 and the
lowest was recorded at P1.The concentrations of Lead across all the sampling stations is well within EPA Sediment Quality guideline. The sources of lead may be of pedogenic background
an essential growth element for plants and animals but at elevated levels it is toxic to some species of aquatic life [40]. In addition, Zn is involved in a variety of enzyme systems which contribute to energy metabolism, transcription and translation. Zinc is also potentially hazardous and excessive concentrations in soil lead to phytotoxicity as it is a weed kill [39] . Zinc is used in galvanizing steel and iron products. Zinc carbonates are used as pesticides.
In the present study the concentration of Zinc varied from
14.3-29.3 ppm. Highest concentration was recorded at P10 and the lowest was recorded at P5. The concentration is well below the EPA sediment quality guidelines and it can be concluded that the sediments are relatively unpolluted.
Nickel:
The larger part of all nickel compounds that are released to the environment will adsorb to sediment or soil particles and become immobile as a result. In acidic ground however, nickel is bound to become more mobile and it will often rinse out to the groundwater. There is not much information available on the effects of nickel upon organisms other than humans. W e do know that high nickel concentrations on sandy soils c an clearly damage plants and high nickel concentrations in surface waters can diminish the growth rates of algae. Micro organisms can also suffer from growth decline due to the presence of nickel, but they usually develop resistance to nickel after a while.
In the present study, the concentration of Nickel varied from 6.1-
22.4 ppm. Highest concentration was recorded at P5 and the lowest was recorded at P1. Comparing to sediment quality guidelines of EPA the concentration of Zinc is below the standard levels and the sediments are relatively unpolluted.
Statistical Analysis:
Pearson Correlation analysis was done to understand the relationship between the heavy metals, their sources and the factors
acting upon their concentration in sediments(Table No 6). In present study correlation matrix was useful to confirm some new associations between metals .Thus, Cu ,Zn and Pb were highly correlatable, which shows that Cu content in sediment was not only due to its presence in the parent rocks but also due to anthropogenic effluents of industrial area, and confirms the combination of metal affiliation of varied origin . Besides Pb also correlated with Ni. It has been shown that the concentration of Pb in sediments is contribution of effluents from a particular industry involving manufacture of paints and pigments and on the other hand, Ni may result from a variety of industrial activities.
Conclusion:
Kabini River is a good example of a site where contributions of pollutants both from natural (lithogenic) sources and anthropogenic
activities. The major pollutants released from industrial activity measures about multiple quantities than those of natural sources of pollutants. The major sources of pollution of the Kabini River are the industrial effluents, return flows, agricultural runoff, municipal and domestic sewage besides pedogenic background contributions. During the study period, the sediment samples during monsoon season, showed significantly lower values than pre monsoon and post monsoon season samples.
The physico-chemical analysis of sediment samples of
Kabini River showed an optimum pH in the suitable range for most of the biological life because the reactions in the acidic range to slightly alkaline is most favorable. Electrical Conductivity (EC) indicates the
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 791
ISSN 2229-5518
presence of significant amounts of anions and cations and during the present study Electrical Conductivity values of sediment samples were found to be in unsafe range. The organic carbon (OC) (%) recorded during the study period, suggested that river is getting organic loads from sewage water which is directly mixing into the river.
The presence of significant quantities of Phosphates, Sulphates and Nitrates in sediment samples indicated that, the river water is getting polluted day day due to discharging of industrial effluent along with the sewage and agricultural runoff to the river system. Contamination primarily occurs because many chemicals bind to organic or inorganic particles that eventually settle to the bottom of rivers and reservoirs. Once contaminants are bound to a particle surface or sorb into its interior matrix, they become less likely to be bio- transformed and desorption is usually very slow; therefore, sorbed contaminants will reside for long periods in the sediment. This is promoted largely by the very high surface area of these particles and the tendency for higher concentrations of organic matter in the fine particles that absorb organic contaminants. Heavy metal pollution is one such problem which is increasing day by day across the world. Incidence of heavy metal accumulation in fish, oysters, sediments and
other components of aquatic ecosystems have been reported from all over the world.
The heavy metals entering the ecosystem may lead to geoaccumulation, bioaccumulation, biomagnification and may have possibilities for environmental transformation into more toxic form. These toxic heavy metals entering in aquatic environment are adsorbed onto particulate matter, although they can form free metal ions and soluble complexes that are available for uptake by biological organisms. Once deposited, binding by sulphides and/ or iron hydroxides immobilizes trace metals until a change in redox or pH occurs. These heavy metals are sensitive indicators for monitoring changes in the water environment. In the present study six heavy metals concentrations in the river sediments were analysed and the data obtained only copper concentration was above the standards of USEPA classification and so also chromium which is slightly above WHO standard for sediment quality. The concentrations of both the metals were high than the established standards near the sampling stations very close to industrial area. It is clear from the study that effluents are getting mixed with the river causing heavy metal pollution in the river kabini.
References:
1. Abowei, J.F.N. and F.D. Sikoki, (2005).W ATER POLLUTION MANAGEMENT AND CONTROL, Double Trust Publications Company, Port
Harcourt; ISBN: 978-30380-20-16,pp: 236.
2. Ajao, F.A. and S.O. Fagade, (1991). A STUDY OF SEDIMENT COMMUNITIES IN LAGOS LAGOON, Nigeria. J.OilChem. Pollut., 7: 85-105.
3. APHA AWWA, WEF (1998) Standard METHODS FOR THE EXAMINATION OF WATER AND WASTEWATER 20TH EDITION. American
Public Health Association, American W ater W ork Association, W ater Environment Federation, W ashington, DC
4. Atabatele, O.E., O.A. Morenike and O.A. Ugwumba, (2005). Spatial VARIATION IN PHYSICO-CHEMICAL PARAMETERS AND BENTHIC INVERTEBRATE FAUNA OF RIVER OGUNPA, IBADAN. The Zoologist, 3: 58-67.
5. Abdel-Shafy HI, Abdel-Basir SE (1991). CHEMICAL TREATMENT OF INDUSTRIAL W ASTEWATER. Environ. Manage. Health, 2: 19-23.
6. Abdel-Ghani .N.T., and Elchaghaby, G. A., (2007), INFLUENCE OF OPERATING CONDITIONS ON THE REMOVAL OF CU , ZN, CD AND PB IONS FROM W ASTE WATER BY ADSORPTION. Int. J. Environ. Sci. Tech., c4 (4), 451 - 456.
7. Adebisi S.A., Ipinromiti K.O., Amoo I.A. (2007). HEAVY METALS CONTENTS OF EFFLUENTS AND RECEIVING W ATERS FROM VARIOUS INDUSTRIAL GROUPS AND THEIR ENVIRONS. J. Appl. Sci., 2(4): 345-348.
8. Adriano D C (2001). TRACE ELEMENTS IN TERRESTRIAL ENVIRONMENTS: BIOCHEMISTRY, BIOAVAILABILITY AND RISKS OF METALS, Springer Verlag, p. 867.
9. Amman, A. A., Michalke, B., and Schramel, P., (2002), SPECIATION OF HEAVY METALS IN ENVIRONMENTAL W ATER BY ION CHROMATOGRAPHY COUPLED TO ICP-MS. Anal. Biochem., 372(3), 448 -452
10. Akcay, H., Oguz, A., and Karapire,C., (2003), Study of HEAVY METAL POLLUTION AND SPECIATION IN BUYAK MENDERES AND GEDIZ RIVER SEDIMENTS. Water Res., 37(4), 813-822.
11. Al-Shiwafi, N., Rushdi, A. I., and Ba-Issa, A., (2005), TRACE METALS IN SURFACE SEAW ATERS AND SEDIMENTS FROM VARIOUS HABITATS OF THE RED SEA COAST OF YEMEN, Environmental Geology, 48(4-5), 590-598.
12. Barnes, R.D. and S. Hughes, (1988). AN INTRODUCTION TO MARINE ECOLOGY. 2nd Edition., Blackwell Scientific Publications, UK., pp:
351.
13. Bakare A.A., Lateef. A, Amuda .O.S., Afolabi. R.O. (2003). THE AQUATIC TOXICITY AND CHARACTERIZATION OF CHEMICAL AND MICROBIOLOGICAL CONSTITUENTS OF W ATER SAMPLES FROM OBA RIVER, ODO-OBA, NIGERIA.
14. Braide, S.A., W .A.L. Izonfuo, P.U. Adakwu, A.C. Chinda and C.C. Obinwo, (2004). W ATER QUALITY OF MINIW EJA STREAM, A SWAMP FOREST STREAM RECEIVING NON-POINT SOURCE W ASTE DISCHARGE IN EASTERN NIGER DELTA, NIGERIA. Sci. Afric.,3(1): 1-8.
15. Brook, J.R., Samson, P. J., and Sillman, S., (1993), THE RELATIONSHIP BETW EEN UPWIND SO2 EMISSIONS AND SO4
CONCENTRATIONS IN PRECIPITATION AT SIX SITES IN THE EASTERN U.S.A., ATMOSPHERIC ENVIRONMENT. Part A. General
Topics, 27(11), 1765-1779.
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 792
ISSN 2229-5518
16. Bruins, M.R., Kapil, S., and Oehme, F.W ., (2000), MICROBIAL RESISTANCE TO METALS IN THE ENVIRONMENT, Ecotox. Environ. Safe.
45(3), 198-207.
17. Cook .N.H, W ells.P.G. (1996). T oxicity OF HALIFAX HARBOUR SEDIMENTS: AN EVALUATION OF MICROTOX SOLID PHASE TEST.
W ater Qual. Res. J. Canada., 31 (4): 673-708.
18. Casper s. T., Mehra a., Farago M. E., Gill R. A.,( 2004) CONTAMINATION OF SURFACE SOILS,RIVER W ATER AND SEDIMENTS BY TRACE METALS FROM COPPER PROCESSING INDUSTRY IN THE CHURNET RIVER VALLEY, STAFFORDSHIRE, UK, Environmental Geochemistry and Health, 26, 59.
19. Chen M., Ma L. Q.,( 2001) COMPARISON OF THREE AQUA REGIA DIGESTION METHODS FOR TW ENTY FLORIDA SOILS, Soil. Sci.
Soc. Am. J., 65, 491.
20. Clesceri, L.S., (1998), STANDARD METHODS FOR THE EXAMINATION OF W ATER AND W ASTE WATER, IN A.E. GREENBERGY AND A.D. EATON (EDS.), COLLECTION AND PRESERVATION OF SAMPLES AND METALS. W ASHINGTON DC: APHA, AWWA, WEF, pp.1-
27–1-35, 3-1–3-21 .
21. David, R.L., L.P. David and W .E. Kenneth, (1981). VARIABLE EFFECTS OF SEDIMENT ADDITION ON STREAM BENTHOS. Hydrobiologia,
79: 187-194.
22. Harikumar, P. S., Nasir, U. P., and Mujeebu Rahman, M.P., (2009), DISTRIBUTION OF HEAVY METALS IN THE CORE SEDIMENTS OF A TROPICAL W ETLAND SYSTEM. Int. J. Environ. Sci. Tech., 6 (2), 225-232.
23. Ikomi, R.B., F.O. Arimoro and O.K. Odihirin, (2005). COMPOSITION, DISTRIBUTION AND ABUNDANCE OF MACROINVERTEBRATES OF THE UPPER REACHES OF RIVER ETHIOPE, DELTA STATE, NIGERIA. The Zoologist, 3: 68-81.
24. Jhingran, V. G., (1991). FISH AND FISHERIES OF INDIA, 3rd ed. Hindustan Publishing Corporation, Delhi, India, PP: 727
25. Khan, R.N., N. Aravindan and C. Kalvati, (2003). DISTRIBUTION OF TW O POST LARVAL SPECIES OF COMMERCIAL PRAWNS (FENNEROPENAEUS INDICUS AND PENAEUS MONODON) IN A COASTAL TROPICAL ESTUARY. J. Aquatic. Science 16(2): 99-104.
26. Lamberson. J.O, Dewitt .T.H, Swartz .R.C.(1992). ASSESSMENT OF SEDIMENT TOXICITY TO MARINE BENTHOS. IN: BURTON GA (ED): SEDIMENT TOXICITY ASSESSMENT, Lewi Pub, Boca Raton, FL, pp. 183-211
27. Larson, T.E., (1971) ORROSIO PHE OME A AUSES A D URES I : W A ER QUALI Y A D REA ME ’, A HA DBOOK OF
PUBLIC W ATER SUPPLIES. 3rd edition. New York, McGraw-Hill Publishing Co.
28. Lee, B.G., Griscom, S.B., Lee, J.S., Choi, H.J., Koh, C.H., Luoma, S.N., and Fisher, N.S., (2000), INFLUENCE OF DIETARY UPTAKE AND REACTIVE SULFIDES ON METAL AVAILABILITY FROM AQUATIC SEDIMENTS, Science, 287(5451): 282-84.
29. Linnik P. M., Zubenko B.,(2000) ROLE OF BOTTOM SEDIMENTS IN THE SECONDARY POLLUTION OF AQUATIC ENVIRONMENTS BY HEAVY-METAL COMPOUNDS, Lakes & Reservoirs Research and Management.
30. Mohan, M., and Kumar, S., (1998), REVIEW OF ACID RAIN POTENTIAL IN INDIA: FUTURE THREATS AND REMEDIAL MEASURES.
Current Science, 75(6), 579-593.
31. Miller .C. V., Foster, G. D., and Majedi, B. F., (2003), BASE FLOW AND STORM FLOW METAL FLUXES FROM TWO SMALL AGRICULTURAL CATCHMENTS IN THE COASTAL PLAIN OF CHESAPEAKE BAY BASIN, UNITED STATES. Appl. Geochem., 18 (4), 483-
501.
32. Nadal. M., Schuhmacher.M, Domingo. D.L. (2004). METAL POLLUTION OF SOILS AND VEGETATION IN AN AREA WITH PETROCHEMICAL INDUSTRIES. Sci. Total Environ., 321(1-3): 59-69.
33. Magalhaes C, Coasta J, Teixeira C, Bordalo AA (2007). IMPACT OF TRACE METALS ON DENITRIFICATION IN ESTUARINE SEDIMENTS OF THE DOURO RIVER ESTUARY, PORTUGAL. Marine Chemistry, 107:332-341.
34. Odiete W .O. (1999). Impacts ASSOCIATED W ITH WATER POLLUTION IN ENVIRONMENTAL PHYSIOLOGY OF ANIMALS AND POLLUTION. Diversified Resources Ltd, Lagos 1st edition, pp. 187-219.
35. Paar, A., (1998), MICROW AVE SAMPLE PREPARATION SYSTEM – INSTRUCTION HANDBOOK AUSTRIA: Anton Paar GmbH, p.128.
36. Ricking M., Terytze K.,( 1999) TRACE METAL AND ORGANIC COMPOUNDS IN SEDIMENT SAMPLES FROM RIVER DANUBE IN RUSSE AND LAKE SREBARNA (BULGARIA), Environmental Geology, 37, 40.
37. Sandroni V., Smith M. M. C., Donovan A. Microwave digestion of sediment and urban particulate matter for trace metal analysis , Talanta, 60,
715, 2003
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 793
ISSN 2229-5518
38. Sachdev, S.L., and W est, P.W ., (1970), CONCENTRATION OF TRACE METALS BY SOLVENT EXTRACTION AND THEIR DETERMINATION BY ATOMIC ABSORPTION SPECTROPHOTOMETER ,Environmental Science & Technology, 4(9),749–751.
39. Singh M, Ansari AA, Muller G, Singh IB (1997). HEAVY METALS IN FRESHLY DEPOSITED SEDIMENTS OF GOMTI RIVER (A TRIBUTARY OF THE GANGA RIVER): EFFECTS OF HUMAN ACTIVITIES. Environ. Geol. 29 (3-4): 246-252.
40. Singare, P.U., Lokhande, R.S., and Bhattacharjee, S.S., (2013), ANALYSIS OF THEHEAVY METAL POLLUTANTS IN SEDIMENT SAMPLES COLLECTED FROM THANE CREEK MAHARASHTRA, INDIA, International Journal of Sustainable Society, 5(3), 296-308.
41. Singare, P.U., Lokhande, R.S., and Naik, K.U., (2010), A CASE STUDY OF SOME LAKES LOCATED AT AND AROUND THANE CITY OF MAHARASHTRA, INDIA, W ITH SPECIAL REFERENCE TO PHYSICO-CHEMICAL PROPERTIES AND HEAVY METAL CONTENT OF LAKE W ATER, Interdisciplinary Environmental Review, 11(1), 90-107.
42. Shuhaimi M.O (2008). METALS CONCENTRATION IN THE SEDIMENTS OF RICHARD LAKE, SUDBURY, CANADA AND SEDIMENT TOXICITY IN AN AMPIPOD HYALELLA AZTECA. Environ. Sci. Technol., 1: 34-41.
43. THE ENVIRONMENT (PROTECTION) RULES, (1986) ,SCHEDULE VI 9, GENERAL STANDARDS FOR DISCHARGE OF ENVIRONMENTAL POLLUTANTS
44. United States Environmental Protection Agency (USEPA), (2002). W ATER QUALITY MONITORING FOR COFFEE CREEK (PORTER COUNTY, INDIANA).Retrieved from: http/www.usepa/research.htm.modecode=62- 28-00-00, (Accessed on: September 29, 2006).
45. Vermeulen L. A., W epener V. SPATIAL AND TEMPORAL VARIATIONS OF METALS IN RICHARDS BAY HARBOUR (RBH), SOUTH AFRICA, Marine Pollution Bulletin, 39 (1-12), 304, 199.
46. W oitke P., W ellmitz J., Helm D., Kube P., Lepom P., Litheraty P., (2003) ANALYSIS AND ASSESSMENT OF HEAVY METAL POLLUTION IN SUSPENDED SOLIDS AND SEDIMENTS OF THE RIVER DANUBE, Chemosphere, 51, 633.
47. W eston, D.P., and Maraya, K.A., (2002), Predicting BIOAVAILABILITY AND BIOACCUMULATION W ITH IN VITRO DIGESTIVE FLUID EXTRACTION. Environ. Toxicol. Chem., 21(05):962-971.
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 794
ISSN 2229-5518
TABLE No: 1: Physico-Chemical parameters in Pre Monsoon season
Sampling stations | pH | EC (µs/cm) ( | Ca (mg/kg) | Mg mg/kg) | Na mg/kg) | K (mg/kg) | %Organic carbon | Nitrate P (mg/kg) | hosphate (mg/kg) | Sulphate (mg/kg) |
P 1 | 7.96 | 22.43 | 5.652 | 1.93 | 8.6 | 3.6 | 0.63 | 0.41 | 0.3 | 1.1 |
P2 | 7.6 | 21.42 | 3.274 | 1.10 | 8.3 | 3.2 | 0.22 | 0.27 | 0.4 | 1.4 |
P3 | 7.85 | 15.38 | 2.645 | 0.98 | 9.7 | 3.5 | 0.19 | 0.26 | 0.28 | 1.2 |
P4 | 7.3 | 22.47 | 2.478 | 0.91 | 9.4 | 4.5 | 0.46 | 0.32 | 0.43 | 1.9 |
P5 | 6.65 | 30.60 | 2.525 | 0.94 | 10.9 | 4.5 | 0.66 | 0.34 | 0.21 | 2.1 |
P6 | 6.6 | 24.44 | 2.74 | 1.04 | 10.1 | 3.7 | 0.40 | 2.2 | 0.34 | 2.7 |
P7 | 7.12 | 23.34 | 2.69 | 1.02 | 9.6 | 3.9 | 0.34 | 2.34 | 0.18 | 2.4 |
P8 | 7.15 | 24.21 | 2.24 | 0.85 | 8.2 | 2.9 | 0.32 | 2.84 | 0.15 | 2.8 |
P9 | 7.01 | 21.27 | 2.35 | 0.89 | 8.7 | 3.1 | 0.38 | 1.3 | 0.12 | 2.2 |
P10 | 6.5 | 25.32 | 2.43 | 0.91 | 11.1 | 4.2 | 0.40 | 1.8 | 0.17 | 2.32 |
TABLE No: 2: Physico-Chemical parameters in Monsoon season
Sampling stations | pH | EC (µs/cm) | Ca (mg/kg) | Mg (mg/kg) | Na (mg/kg) | K (mg/kg) | % OC | Nitrate P (mg/kg) | hosphate (mg/kg) | Sulphate (mg/kg) |
P 1 | 7.5 | 21.42 | 5.32 | 1.72 | 8.1 | 3.4 | 0.51 | 0.36 | 0.24 | 0.94 |
P2 | 7.4 | 19.38 | 3.143 | 1.08 | 7.9 | 3.1 | 0.19 | 0.24 | 0.32 | 1.13 |
P3 | 7.39 | 14.28 | 2.472 | 0.86 | 9.4 | 3.24 | 0.18 | 0.23 | 0.21 | 1.0 |
P4 | 7.2 | 20.40 | 2.334 | 0.82 | 9.1 | 3.9 | 0.40 | 0.27 | 0.33 | 1.5 |
P5 | 6.6 | 28.56 | 2.397 | 0.79 | 9.7 | 3.8 | 0.57 | 0.30 | 0.18 | 1.8 |
P6 | 6.42 | 22.36 | 2.483 | 0.97 | 8.8 | 3.0 | 0.36 | 1.9 | 0.27 | 2.0 |
P7 | 7.10 | 21.32 | 2.517 | 0.97 | 8.7 | 3.4 | 0.30 | 2.1 | 0.15 | 1.7 |
P8 | 7.13 | 23.26 | 2.190 | 0.73 | 7.8 | 2.6 | 0.31 | 2.5 | 0.14 | 2.23 |
P9 | 7.10 | 20.24 | 2.216 | 0.81 | 7.9 | 2.7 | 0.32 | 1.1 | 0.10 | 1.6 |
P10 | 6.47 | 24.28 | 2.195 | 0.85 | 10.96 | 3.60 | 0.38 | 1.5 | 0.13 | 2.2 |
TABLE No:3 :Physico -Chemical parameters in Post Monsoon
Sampling stations | pH | EC (µs/cm) | Ca (mg/kg) | Mg (mg/kg) | Na (mg/kg) | K (mg/kg) | %Organic carbon | Nitrate (mg/kg) | hosphate (mg/kg) | Sulphate (mg/kg) |
P 1 | 7.6 | 19.38 | 5.241 | 1.37 | 7.9 | 3.0 | 0.42 | 0.25 | 0.15 | 0.77 |
P2 | 7.35 | 18.36 | 2.898 | 0.98 | 7.6 | 2.8 | 0.18 | 0.15 | 0.21 | 0.85 |
P3 | 7.48 | 13.26 | 2.25 | 0.72 | 8.8 | 2.75 | 0.15 | 0.19 | 0.17 | 0.90 |
P4 | 7.12 | 19.38 | 2.187 | 0.69 | 8.7 | 3.2 | 0.28 | 0.24 | 0.22 | 1.20 |
P5 | 6.54 | 25.50 | 2.221 | 0.70 | 9.1 | 3.4 | 0.42 | 0.22 | 0.15 | 1.60 |
P6 | 6.38 | 22.32 | 2.324 | 0.84 | 8.6 | 2.8 | 0.25 | 1.4 | 0.20 | 1.40 |
P7 | 7.09 | 21.28 | 2.298 | 0.29 | 8.4 | 2.75 | 0.29 | 1.34 | 0.12 | 1.30 |
P8 | 7.11 | 20.20 | 2.02 | 0.65 | 7.2 | 2.5 | 0.26 | 1.54 | 0.11 | 1.90 |
P9 | 6.89 | 22.18 | 2.04 | 0.62 | 7.4 | 2.1 | 0.30 | 1.03 | 0.08 | 1.36 |
P10 | 6.42 | 23.24 | 2.10 | 0.71 | 9.5 | 3.10 | 0.34 | 1.2 | 0.10 | 2.00 |
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 795
ISSN 2229-5518
Table No.4: Mean values of Physico-chemical parameters during the study period
| pH | E.C | Ca | Mg | Na | K | O.C | NO32- | PO42- | SO42- |
P1 | 7.68 | 21.07 | 5.40 | 1.67 | 8.2 | 3.3 | 0.52 | 0.34 | 0.23 | 0.93 |
P2 | 7.45 | 19.72 | 3.35 | 1.05 | 7.93 | 3.03 | 0.19 | 0.21 | 0.31 | 1.12 |
P3 | 7.57 | 14.30 | 2.45 | 0.85 | 9.3 | 3.16 | 0.17 | 0.23 | 0.22 | 1.03 |
P4 | 7.2 | 20.75 | 2.32 | 0.80 | 9.06 | 3.86 | 0.44 | 0.27 | 0.32 | 1.53 |
P5 | 6.59 | 28.22 | 2.37 | 0.81 | 9.9 | 3.9 | 0.51 | 0.28 | 0.18 | 1.83 |
P6 | 6.46 | 23.04 | 2.51 | 0.95 | 9.16 | 3.16 | 0.39 | 1.83 | 0.27 | 2.03 |
P7 | 7.10 | 21.98 | 2.49 | 0.86 | 8.9 | 3.35 | 0.28 | 1.92 | 0.15 | 7.8 |
P8 | 7.13 | 22.55 | 2.15 | 0.85 | 7.73 | 2.66 | 0.32 | 2.29 | 0.13 | 2.31 |
P9 | 7.06 | 21.23 | 2.2 | 0.77 | 8.00 | 2.63 | 0.45 | 2.5 | 0.10 | 1.72 |
P10 | 6.46 | 24.28 | 2.28 | 0.82 | 10.4 | 3.96 | 0.58 | 3.03 | 0.13 | 2.24 |
TABLE No: 5: Total Heavy metal Concentration in Kabini river Sediments
Sampling Stations | Iron | Copper | Chromium | Lead | Zinc | Nickel |
P1 | 4157.3 | 88.4 | 13.9 | 5.1 | 30.4 | 6.1 |
P2 | 4846.4 | 63.2 | 6.8 | 11.8 | 23.6 | 5.7 |
P3 | 5127.1 | 57.6 | 14.3 | 11.3 | 25.4 | 22.4 |
P4 | 4541.6 | 69.8 | 10.6 | 12.4 | 28.6 | 7.4 |
P5 | 6118.1 | 75.6 | 26.1 | 14.6 | 29.3 | 8.1 |
P6 | 5846.7 | 72.3 | 19.3 | 13.2 | 28.7 | 7.8 |
P7 | 4763.3 | 66.4 | 10.3 | 11.4 | 20.4 | 6.4 |
P8 | 4913.2 | 63.9 | 7.6 | 11.6 | 18.9 | 6.8 |
P9 | 5110.9 | 93.9 | 12.7 | 10.9 | 14.3 | 6.5 |
P10 | 4927.5 | 91.3 | 16.7 | 11.1 | 33.1 | 6.9 |
TABLE No: 6: Pearson Correlation co-efficient Matrix
| Iron | Copper | Chromium | Lead | Zinc | Nickel |
Iron | 1 | | | | | |
Copper | -0.10157 | 1 | | | | |
Chromium | 0.730704 | 0.315522 | 1 | | | |
Lead | 0.769458 | -0.39692 | 0.319688 | 1 | | |
Zinc | 0.057208 | 0.139639 | 0.524431 | 0.074454 | 1 | |
Nickel | 0.164791 | -0.45676 | 0.66555 | 0.091176 | 0.064167 | 1 |
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 796
ISSN 2229-5518
TABLE No: 7: USEPA Sediment Quality Guidelines for few heavy metals:
Heavy Metal | Slightly polluted | Polluted | Heavily polluted | Avg. Heavy metal conc.in Kabini |
Copper | <25 | 25-50 | >50 | 74.24 |
Chromium | <25 | 25-75 | >76 | 13.83 |
Lead | <40 | 40-60 | >60 | 11.34 |
Zinc | <90 | 90-200 | >200 | 25.27 |
Nickel | <20 | 20-50 | >50 | 8.41 |
9
8
7
6
5
4
3
2
1
0
1 2 3 4 5 6 7 8 9 10
Sampling stations
Pre Monsoon
Monsoon
Post Monsoon
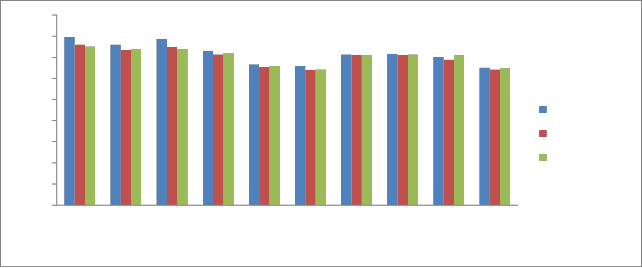
Fig 2. Variations in pH of Sediments
35
30
25
20
15
10
5
0
1 2 3 4 5 6 7 8 9 10
Sampling stations
Pre Monsoon
Monsoon
Post Monsoon
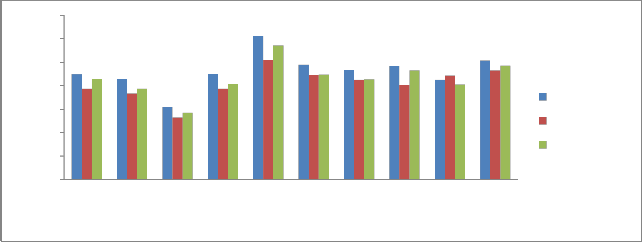
Fig 3. Variations in E C of Sediments
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 797
ISSN 2229-5518
6
5
4
3
2
1
0
1 2 3 4 5 6 7 8 9 10
Sampling stations
Pre monsoon
Monsoon
Post monsoon
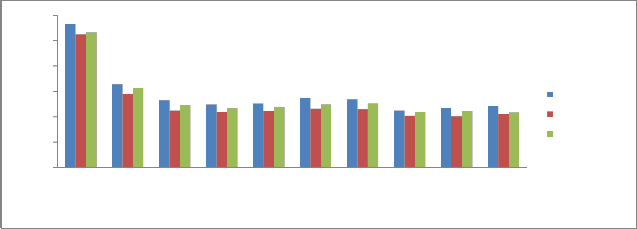
Fig 4. Variations in Calcium concentrations of Sediments.
2.5
2
1.5
1
0.5
Pre monsoon
Monsoon
Post Monsoon
0
1 2 3 4 5 6 7 8 9 10
Sampling stations

Fig 5. Variations in Magnesium concentrations of Sediments.
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 798
ISSN 2229-5518
12
10
8
6 pre Monsoon
4 Monsoon
Post Monsoon
2
0
1 2 3 4 5 6 7 8 9 10
Sampling stations

Fig 6. Variations in Sodium concentrations of Sediments.
5
4
3
Pre Monsoon
2 Monsoon
1 Post Monsoon
0
1 2 3 4 5 6 7 8 9 10
Sampling stations
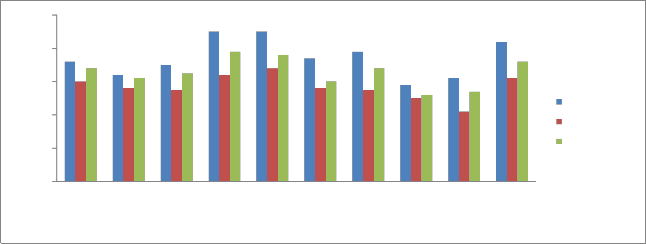
Fig 7. Variations in Potassium concentrations of Sediments.
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 799
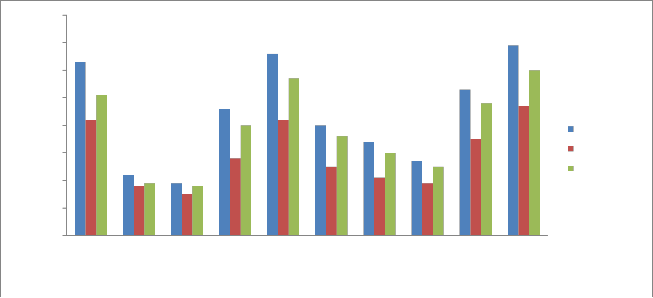
ISSN 2229-5518
0.8
0.7
0.6
0.5
0.4
0.3
0.2
Pre monsoon monsoon
Pre monsoon
0.1
0
1 2 3 4 5 6 7 8 9 10
Sampling stations
Fig 8.Variations in Organic Carbon (%) of Sediments.
4.5
4
3.5
3
2.5
2
1.5
1
0.5
0
1 2 3 4 5 6 7 8 9 10
Sampling stations
Pre Monsoon
Monsoon
Post Monsoon
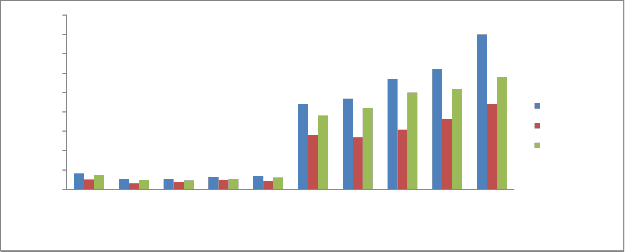
Fig 9. Variations in Nitrate concentrations of Sediments.
IJSER © 2013
http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 800
ISSN 2229-5518
0.5
0.45
0.4
0.35
0.3
0.25
0.2
0.15
0.1
0.05
0
1 2 3 4 5 6 7 8 9 10
Sampling stations
Pre monsoon
Monsoon
Post Monsoon
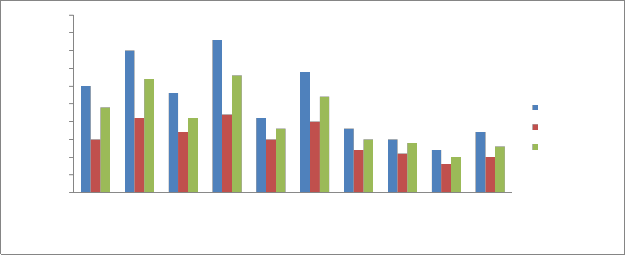
Fig 10. Variations in Phosphate concentrations of Sediments.
3
2.5
2
1.5
1
0.5
Pre monsoon
Monsoon
Post Monsoon
0
1 2 3 4 5 6 7 8 9 10
Sampling stations
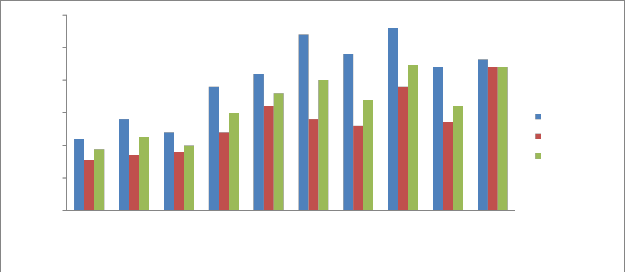
Fig 11. Variations in Sulphate concentrations of Sediments.
IJSER © 2013
http://www.ijser.org