International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1552
ISSN 2229-5518
Fattah . M. K.1, Dahab, K. A. 2 and Ahmed,M. G1
1-Environmental Studies and Research Institute, University of Sadat city, Egypt.
2- Faculity of science, Menofiya University, Egypt.
* Corresponding author: Dr: Mohamed Kame Fattah
Environmental Studies and Research Institute (ESRI), University of Sadat city, Egypt. E-mail: kamelhydro90@yahoo.com. , mohamed.fattah@esri.usc.edu.eg
During the last decade, the continuous development of human society as well as the side effects of land reclamation projects left negative impacts on water resources in Nubaria area, west of Nile Delta area. Such negative impacts are pronounced in continuous groundwater deterioration and water pollution.
The present work focus on the environmental impacts on geochemical evolution of the Quaternary aquifer in the study area and determine geochemical type, origin, evolution of groundwater and their relation to the prevailing hydrogeological and environmental conditions, and finally evaluate the suitability of ground water for different uses. Most of groundwater samples are good to moderate water class and suitable for irrigation purposes.
The area of study is a part of the western Nile
Delta fringe. This area is limited by latitudes 30º 15′
- 30º 50′ N and longitudes 30º 00′ - 30º 48′ E, with area of about 3100 km2. It is bounded by El Nubariya canal from the northeast, El Nasr canal from the northwest, El Rayah El Naseri from the east and Cairo – Alex. desert road from the south
(Fig. 1). The chemistry of groundwater is an essential parameter for assessing the environmental characteristics of an area [1]. The main factors affecting water quality changes are lithofacies geographical conditions, groundwater recharge and
runoff conditions, and the degree of openness of
groundwater systems [2] .Hydrochemical evolution is commonly used to reconstruct geochemical evolution of groundwater from one point in an aquifer to another point located in the inverse direction along the groundwater flow path [3]. Nile Delta area, is one of the most important areas for the land living. Accordingly, extensive need to the water as a supplement for irrigation and domestic use attracted more attention, and great efforts were established to evaluate water resources in the investigated area. Variation of groundwater quality in an area is a function of physical and chemical parameters that are greatly influenced by geological formations and anthropogenic activities [4].
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1553
![]()
![]()
![]()
![]()
![]()
![]()
![]()

![]()
![]()
![]()
![]()
![]()
![]()
ISSN 2229-5518
30 | 00 30 | 30 31 | 00 |
50 | Mediterranean Sea 82 31 81 7890 18 30 Rosetta 1167966653 Damietta 614165 Lake Idku Kafr El Sheikh 6291 61 71 31 Damanhur El Mansoura 7720 2607 60 62 59 55 00 Abu El Matamir 743 83 Tanta Kafr Bolin 35 34 568 5 33 14 12115017 Shibin 30 El Kom Kafr 32 30 Dawoud 24 El Sadat City Banha 2758 36 84 El Khatatba 26 3130 The Study Area 77 1 51 85 30 86 592 00 Km. Cairo 29 76 0 10 20 30 227352 834 75 30 00 627 30 00 30 31 31 30 2 37 53 54 50 49 43 40 44 39 48 38 41 47 42 45 46 Legand Main Road Main irrigation Canal Secondary irrigation Canal 0 4 12 20 Km. Shallow well ( 65 m ) Deep well ( 65 m - 170 m ) Scale |
| |
30 30 | Mediterranean Sea 82 31 81 7890 18 30 Rosetta 1167966653 Damietta 614165 Lake Idku Kafr El Sheikh 6291 61 71 31 Damanhur El Mansoura 7720 2607 60 62 59 55 00 Abu El Matamir 743 83 Tanta Kafr Bolin 35 34 568 5 33 14 12115017 Shibin 30 El Kom Kafr 32 30 Dawoud 24 El Sadat City Banha 2758 36 84 El Khatatba 26 3130 The Study Area 77 1 51 85 30 86 592 00 Km. Cairo 29 76 0 10 20 30 227352 834 75 30 00 627 30 00 30 31 31 30 2 37 53 54 50 49 43 40 44 39 48 38 41 47 42 45 46 Legand Main Road Main irrigation Canal Secondary irrigation Canal 0 4 12 20 Km. Shallow well ( 65 m ) Deep well ( 65 m - 170 m ) Scale |
| |
10 30 | Mediterranean Sea 82 31 81 7890 18 30 Rosetta 1167966653 Damietta 614165 Lake Idku Kafr El Sheikh 6291 61 71 31 Damanhur El Mansoura 7720 2607 60 62 59 55 00 Abu El Matamir 743 83 Tanta Kafr Bolin 35 34 568 5 33 14 12115017 Shibin 30 El Kom Kafr 32 30 Dawoud 24 El Sadat City Banha 2758 36 84 El Khatatba 26 3130 The Study Area 77 1 51 85 30 86 592 00 Km. Cairo 29 76 0 10 20 30 227352 834 75 30 00 627 30 00 30 31 31 30 2 37 53 54 50 49 43 40 44 39 48 38 41 47 42 45 46 Legand Main Road Main irrigation Canal Secondary irrigation Canal 0 4 12 20 Km. Shallow well ( 65 m ) Deep well ( 65 m - 170 m ) Scale | 30 30 | |
30 | Mediterranean Sea 82 31 81 7890 18 30 Rosetta 1167966653 Damietta 614165 Lake Idku Kafr El Sheikh 6291 61 71 31 Damanhur El Mansoura 7720 2607 60 62 59 55 00 Abu El Matamir 743 83 Tanta Kafr Bolin 35 34 568 5 33 14 12115017 Shibin 30 El Kom Kafr 32 30 Dawoud 24 El Sadat City Banha 2758 36 84 El Khatatba 26 3130 The Study Area 77 1 51 85 30 86 592 00 Km. Cairo 29 76 0 10 20 30 227352 834 75 30 00 627 30 00 30 31 31 30 2 37 53 54 50 49 43 40 44 39 48 38 41 47 42 45 46 Legand Main Road Main irrigation Canal Secondary irrigation Canal 0 4 12 20 Km. Shallow well ( 65 m ) Deep well ( 65 m - 170 m ) Scale | 10 | |
30 | 00 30 | 30 31 | 00 |
![]()
![]()
![]()
![]()
![]()
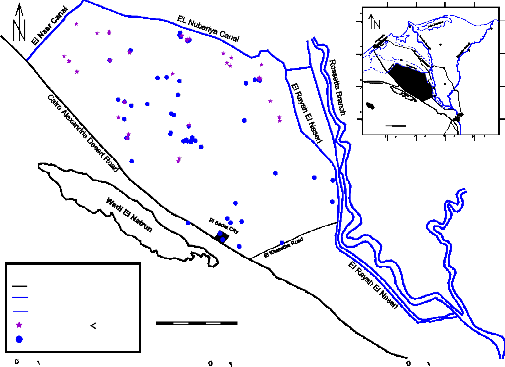
Fig. (1) Location well map for the production wells in the study area
Material and methods
Complete chemical analysis of 50 groundwater samples collected from the study area, (Fig. 1). groundwater analyses were carried according to the methods adopted by [5]. The analyses conducted both in the field and laboratory include total dissolved solids (TDS), measurement of pH, electrical conductivity (EC), concentrations of Ca+2, Mg+2, Na+ and K+ as cations, CO3 -2, HCO3 -, SO4 -2 and Cl- as
anions, and determination of the trace elements Fe3+ ,
Mn 2+, Al+3 and Zn2+. ). The water pH, temperature, electrical conductivity (EC), salinity and total dissolved solids (TDS) were measured in the field. Water samples were filtered at the time of collection using 25 mm puradisc syringe filtration unit of 0.45
µm pore size and acidified to pH 2 with concentrated HNO3 acid and analyzed directly using flame (air- acetylene burner) atomic absorption spectrometry (FAAS – Zeeman AAS Z-5000, Hitachi, Japan)to measure Na+ , K+, Fe3+ , Mn 2+, Al+3 and Zn2+. SO42- was measured using a HACH (DR/2040 – Loveland, CO, USA) meter, Ca2+ ,Mg+2,Cl- and HCO3 2– were analyzed using argentometric and titration methods respectively, where the procedures of analyses adopted in this study were based on the methods described in ASTM (2002). Also, saturation indices were calculated
with the help of NETPATH computer program [6]. A
global positioning system (GPS) Garment 12 was used for location and elevation readings. The NETPATH computer code was used to model the major hydrogeochemical reactions contributing to the evolution of groundwater chemistry in the study area. The model is described in detail by [6]. It can provide a useful insight into the probable processes governing groundwater chemistry [7] and [8]
I.Geological and hydrogeological setting:
1.2 Geological setting
The geology of the considered area and its vicinities concluded that, the rocks of Miocene, Pliocene, and Quaternary times are the most outcropping sediments. The surface geologic structure is relatively simple; however in the subsurface many complicated structures are detected.The study area is covered by extensive exposures of sedimentary successions ranging from Late Cretaceous to Quaternary. The surface geological structure is relatively simple, however many complexities are detected in the subsurface. The subsurface succession is characterized by the occurrence of many unconformity surfaces and considerable faulting and folding structures, which directly affect the groundwater occurrences.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1554
ISSN 2229-5518
Pleistocene aquifer represents the main water resource of water supply for domestic, industrial and irrigation purposes in the study area. The Pleistocene aquifer is mainly composed of fluviatile graded sand and gravel intercalated with clay lenses. The thickness of aquifer increasing in northeast and northern direction, it reaching its minimum values near Wadi El Natrun, El Sadat City, while it reach its maximum values at near Nile Delta aquifer. Groundwater in the study area is under phreatic condition with the exception of some localities, which display a semi- confined condition From hydrogeological cross section was constructed to describe the lithological facies,
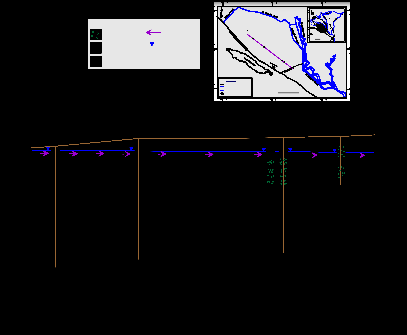
hydrostructural setting and hydrogeological properties of the
aquifer, (Fig. 2).The aquifer is built up of sand, gravel and clay deposits belonging to the Quaternary time (Holocene and Pleistocene). Environmentally, the Pleistocene sediments belong to Deltiac facies composed of gravelly sands and sands intercalated with thin clay layers and capped by lagoonal facies composed of sands, clay and gypsiferous clay .The saturated thickness of the aquifer in the study area shows variable values, it increases gradually in the northeast direction , it ranges from 100 m to 200 m in the southwestern portion near Cairo-Alex. Desert Road and from 260 m to 280 m at El-Sadat City, while it reaches its maximum values at the northeastern part ranging from 300 m to 320 m.
30 00
30 30 31 00
Mediterranean Sea
31
30
Rosetta
Damietta
Legand:
Lake Idku
31
00 Abu El Matamir
Damanhur
Kafr El Sheikh
Tanta
El Mansoura
Kafr Bolin
Gravelly sand
Flow direction C
30
30
The Study Area
El Sadat City
Shibin
El Kom
Kafr
Dawoud
El Khatatba
Banha
W 18
30
00 Km.
0 10 20 30
Cairo
sand
Clay
Water table
W 17
W 16
W 15
W 14
C
30 00 30 30 31 00 31 30
Legand
Main Road
Main irrigation Canal
Secondary irrigation Canal
0 4 12 20 Km.
(M.)
100
0.0
- 200
Pleistocene
Pliocene
30 00
Pleistocene
Pliocene
Area of study
30 30
Scale
31 00
Pleistocene
100
0.0
- 300
- 300
The depth to groundwater varies less than one meter near El Nubariya canal (northern parts) to about
20 m. at El Bustan area (central part), while it exceeds
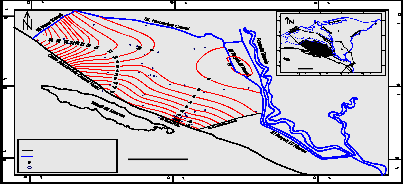
60 m below the ground surface in Sadat City and its vicinities(southern part).Groundwater levels ranges from + 5 m to + 4 m (amsl) in the northern part and
from (–4 m) and (– 5 m) (bmsl) at the southern part (Fig.3). The low values of groundwater levels recorded at the southern part reflect impact of large scale groundwater extraction rates and limited groundwater recharge to these localities.
30 00
30 30 31 00
16 9
Mediterranean Sea
31
30
Rosetta
Damietta
1110
55
54
1132
22
21
6
78
57
20
15 51
Lake Idku
31
00 Abu El Matamir
30
30
The Study Area
Damanhur
Kafr Bolin
El Sadat City
Kafr El Sheikh
Tanta
Shibin
El Kom
Kafr
Dawoud
El Khatatba
Banha
El Mansoura
19
53
14 18
5
4 1 2
3 17
30
00 Km.
58 0 10 20 30
30 00
30 30
31 00
Cairo
31 30
49
56 50
48
47
52
26
43
42
41
40
25
24 46
28
23 45
29 321 27
39 30
3536 33
38
44
34
Legand
Main Road
Main irrigation Canal
Water level contour line
Observation well
0 4 12 20 Km.
Scale
30 00
30 30
31 00
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1555
ISSN 2229-5518
(Fig. 3): Water levels contour map of the Pleistocene aquifer in the study area
The majority of samples in shallow groundwater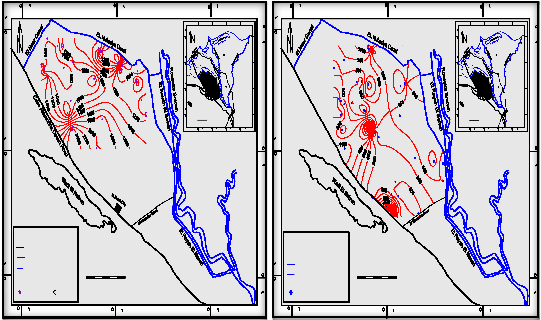
wells (less than 65m)of the Pleistocene aquifer in the
study area have salinity content ranging from 1024 mg/l to 2688 mg/l represented in northern and southern parts of the study area (Fig. 3)
30 00
30 30 31 00
30 00
30 30 31 00
Mediterranean Sea
31
30
Rosetta
Damietta
Mediterranean Sea
31
30
Rosetta
Damietta
Lake Idku
31
00 Abu El Matamir
Damanhur
Kafr El Sheikh
El Mansoura
Lake Idku
31
00 Abu El Matamir
Damanhur
Kafr El Sheikh
El Mansoura
Tanta
Tanta
Kafr Bolin
Kafr Bolin
30
30
El Sadat City
Shibin
El Kom
Kafr
Dawoud
El Khatatba
Banha
30
30
El Sadat City
Shibin
El Kom
Kafr
Dawoud
El Khatatba
Banha
The Study Area
30
00 Km.
0 10 20 30
30 00
30 30 31 00
Cairo
31 30
The Study Area
30
00 Km.
0 10 20 30
30 00
30 30
31 00
Cairo
31 30
Legand
Main Road
Main irrigation Canal
Secondary irrigation Canal
800 Salinity contour line
Shallow well ( 65 m )
30 00
0 4 12 20 Km.
Scale
30 30
31 00
Legand
Main Road
Main irrigation Canal
Secondary irrigation Canal
500 Salinity contour line
Deep well ( 65 m - 170 m )
30 00
0 4 12 20 Km.
Scale
30 30
31 00
The majority of groundwater samples of deep wells (more than 65m ) ranging from 320 mg/l a the south to 1200 mg/l at the north. But in some region of the southwestern parts (El Sadat City and Cairo - Alex. Desert Road) display relatively high salinity ranging from
1200 to 2253 mg/l (Appendix I), the increase in salinity contents that recorded at these parts reflect the over pumping and leaching processes of salts from clay present in the aquifer materials. The relationship between salinity contents and the other ions in deep wells indicates that sodium, calcium, potassium, sulphate and chloride are the effective ions which caused an increase of water salinity and change in groundwater quality. Salinity displays good linear relationship with calcium, sodium, sulphate and chloride ions with high correlation coefficients 0.80, 0.96,
0.93 and 0.95 respectively, moderate linear relationship with potassium ions with correlation coefficient 0.58, poor linear relationship with magnesium and bicarbonate with correlation coefficient 0.26 and 0.31 respectively.
II.2- Hypothetical salts
Regarding hypothetical salt combinations of the Pleistocene aquifer in the study area, groundwater samples have the following assemblage:
Assemblage I: NaCl, NaR2RSOR4R, Na (HCOR3R)R2R, Mg
(HCOR3R)R2R, Ca (HCOR3R)R2R
(17% of the total groundwater samples) Assemblage II: NaCl, NaR2RSOR4R, MgSOR4R,R R Mg (HCOR3R)R2R, Ca (HCOR3R)R2
R R(37% of the total groundwater
samples)
Assemblage III: NaCl, NaR2RSOR4R,R RMgSOR4R, CaSOR4R , Ca
(HCOR3R)R2
(44% of the total groundwater samples) Assemblage IV: NaCl, MgClR2R, MgSO4,R R CaSOR4R , Ca (HCOR3R)R2
(2% of the total groundwater samples)
Most groundwater samples of the Pleistocene aquifer are characterized by the assemblages of salt combination (II and III); assemblage II (37% of the total groundwater samples) represents an earlier stage of chemical development than that of assemblage III. Assemblages I and II (two bicarbonate salts) reflects the effect of pure rain water or surface water on groundwater and also reflects recent recharge. Assemblage III (three sulphate salts) reflects the effects of leaching and dissolution of evaporates deposits and represent intermediate stages of chemical development. Very few groundwater samples (2% of
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1556
ISSN 2229-5518
the total groundwater samples) are characterized by assemblage IV which represents an advanced stage of chemical development, this salt assemblage reflect the effect of marine salt pollution (marine facies groundwater type) with possible contribution of cation exchange phenomena resulting from the presence of the clay intercalated with the Pleistocene aquifer in the study area.
II.3-. Hydrochemical coefficients:
The hydrochemical coefficient gave good indication of geochemical processes occurring in groundwater solution and groundwater origin (Appendix IV). The hydrochemical coefficients (rNa/rCl), (rCa/rMg), (rMg+rCa/rNa), (rSO4/rCl) and (rHCO3/rCl) were calculated (appendix VII).
The value of the hydrochemical coefficient (rNa/rCl) of groundwater of the Pleistocene aquifer varies from 1.17 (sample No. 60) to 6.95 (sample No. 70) with an average of 4.06 in shallow groundwater wells and from 0.86 (sample No. 47) to 4.15 (sample No. 20) with an average 2.5 in deep groundwater wells. The excess of sodium over chloride water samples reflects meteoric water origin. [10] concluded that the increasing Na+ ions might have theoretically originated by dissolution of sodium bearing silicates from the country rocks. This is due to the complete flushing of marine deposits or old sea water through movement of rain water that horizontally or vertically infiltrated and settled in siliceous aquifer materials in the past time
The value of the hydrochemical coefficient (rSO4/rCl) varies from 0.59 (sample No. 59) to 5.95 (sample No. 85) with an average of 3.27 of shallow wells. It has values ranging from 0.4 (sample No. 14) to 2.67 (sample No. 35) with an average 1.53 in deep groundwater wells. Predominance of saulphate over chloride in the majority of samples reflect leaching and dissolution of sulphate bearing minerals such as evaporites and Gypsum that dominant in aquifer materials.
The value of the hydrochemical coefficient (rCa/rMg) of the Pleistocene groundwater aquifer samples ranges from 0.33 (sample No. 75) to 6.6 (sample No. 83) with an average of 3.46 in shallow groundwater wells and from 0.13 (sample No. 33) to
5.35 (sample No. 13) with an average 2.74 in deep groundwater samples. The excess of calcium over magnesium in the majority of samples reflect leaching and dissolution of carbonate minerals that dominant in
aquifer materials.
The value of the hydrochemical coefficient (rMg+rCa/rNa) in the Pleistocene groundwater aquifer samples varies from 0.062 (sample No. 69) to
1.28 (sample No. 85) with an average 0.67 in shallow groundwater wells and from 0.08 (sample No. 20) to
1.96 (sample No. 6) with an average 1.02 in deep groundwater samples. This ionic ratio is more than unity in some groundwater samples 9 %), the majority of samples have value less than unity which indicate predominance of sodium over calcium and magnesium as well as leaching processes of sodium bearing minerals such as halite and Sodic - plagioclase that dominant aquifer materials.![]()
r Cl / r (HCO3 - + CO3 -2) ratio
1- Normal good groundwater (less than 1)
2- Slightly contaminated water (more than 1 and less than 2)
3- Moderately contaminated water (2-6)
4- Seriously contaminated water (more than 6 and less than 15)
5- Highly contaminated water (more than 15)
The values for the main aquifer range from
0.178 (well No. 55) to 26.366 (well No. 54) in shallow groundwater wells, and from 0.267 (well No. 46) to
14.334 (well No. 42) in deep groundwater wells. These values are less than that in sea water (220) and more than in rain water (0.68). Most of groundwater samples (31 % shallow wells and 37% deep wells) are considered moderately contaminated water (2-6).This means that the groundwater has a mixed origin that is possibly pure meteoric water affected by trace of marine deposits.
II.4. Geochemical classification of groundwater:
Piper's trilinear diagram [12], this diagram illustrates the trend of geochemical evolution of groundwater through mixing with other sources and through water- rock interaction.
Piper's trilinear diagram for shallow and deep groundwater wells consequently show that (Figs. 5. and
6), the projection of chemical composition of shallow groundwater on the diamond field revealed that most of water chemical compositions are plotted in subarea 7 (32 wells), and in subarea 9 (3 wells) (Fig. 5). Piper's trilinear diagram for deep groundwater wells show that, the
projection of chemical composition of deep groundwater
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1557
ISSN 2229-5518
on the diamond field revealed that most of water chemical compositions are plotted in subarea 7 (42 wells) and in subarea 9 (9 wells), where subarea 9 reflects fresh water and that reflects Na-HCOR3R and Ca-HCOR3R water type,
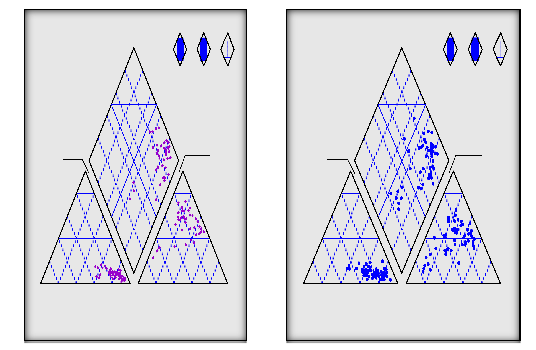
while subarea 7 reflects primary salinity properties
characterized by Na++ + K+ > Cl־ + SO -- and sodium
chloride salts (Fig. 5.26). The appearance of NaCl water type with high percentage of MgSOR4R and CaSOR4R reveals high phase of groundwater mineralization.
100
80 80
6
1 4 9
5 7
2 3 9
8
100
80 80
6
1 4 9
5 7
2 3 9
8
60 60
Subdivisions of the diamond shaped
field of Piper trillinear diagram
60 60
Subdivisions of the diamond shaped
field of Piper trillinear diagram
4
40 40
4
40 40
20 20
20 20
Na + K 0
100
20
80
CO3 + HCO3
0
100
20
80
Na + K 0
100
20
80
CO3 + HCO3
0
100
20
80
40 40
60
Mg
60 60
40
20 80 80
60
SO4
40
20
40 40
60
Mg
60 60
40
20 80 80
60
SO4
40
20
100 80
60 40 20
Ca
100
0 0
20 40 60
Cl
0
80 100
100 80
60 40 20
Ca
100
0 0
20 40 60
Cl
0
80 100
Cations Anions
Cations Anions
NETPATH was used to environmental simulate net geochemical mass-balance reactions between initial and final waters along a hydrologic flow path. The program uses chemical data for waters samples. The processes of dissolution, precipitation, ion exchange, oxidation/reduction, degradation of organic compounds, incongruent reaction, gas exchange, mixing, evaporation, dilution can be considered. This program simulates selected evolutionary waters for every possible combination of the plausible phases that account for the composition of a selected set of chemical constraints in the system (Table 2 ). The NETPATH software includes a data-base program (DB) for storing and editing chemical data for use in NETPATH. A mass-balance is defined as the masses (per kilogram H2O) of a set of plausible minerals and gases that must enter or leave the initial solution in order to define exactly a set of selected elemental, electron transfer, and isotopic constraints observed in a
final (evolutionary) water. In hydrochemical systems the number of reacting phases is often larger than the number of constraints necessary to define their composition. NETPATH solves a set of linear equations that account for conservation of mass, and (optionally) conservation of electrons and selected isotopes to find every subset of the selected phases (every model) that satisfies the chosen constraints. Each mass-balance model can be treated as an isotope evolution problem solving isotope mass balance and Raleigh distillation problems to predict the isotopic composition at the final point on the flow path.
5.4.1. Input data:
Water analyses are entered through the interactive data base (DB) program. DB allows editing and processing of the data through the WATEQF equilibrium model [13], [14] and [15] to produce an input file (filename. PAT) to NETPATH DB (and NETPATH) accept laboratory and field analytical data for waters containing the elements Na, K, Ca, Mg, Cl, S, C, Fe, Mn, N and P. Temperature and pH are required for every water analysis, the rest are optional.
All files and data used by DB are interactively updated
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1558
ISSN 2229-5518
and maintained. DB produces full speciation output for all waters in the format of WATEQF results (Fig.
13 ), maintains the [filename]. LON data file of water analyses and produces the [filename]. PAT input file to NETPATH. Files for checking charge imbalances in original data and data reports of water analyses are optionally produced from DB. NETPATH automatically updates the minerals data file, NETPATH.DAT, upon normal termination, saves model input files and model result files.
Samples were analyzed and the major anions and cations were processed into the program WATEQF as an input file for each sample. The program converts the concentrations into mol/l and calculates the activities of elements and species of each sample. The program calculates the saturation indices of Calcite, Aragonite, Dolomite, Siderite, Rhodochr, Gypsum, Anhydrite, Hematite, Goethite, Vivianit, H2 Gas and Partial carbon dioxide P-CO2. The positive signs of the saturation index (SI) mean that the solution is supper saturated and the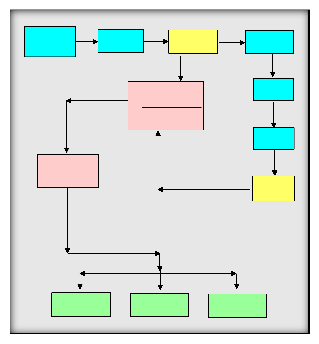
negative signs reflect that the solution is sub-saturated and
zero value means that the solution is in equilibrium state. The saturation state is calculated as follows:
Ω = IAP / K Where, IAP = Ion Activity Produc K
= solubility product
If Ω > 1, the supersaturating state prevails,
Ω < 1, the sub-saturating state prevails, Ω = 1, the
equilibrium state prevails.
For the large deviations from the equilibrium a logarithmic scale can be useful like the saturation index (SI). The index is expressed as:
SI = Log (IAP/K)
If SI = 0, there is equilibrium state between the minerals and the solution.
SI < 0, there is sub-saturation state prevails,
SI > 0, there is supersaturating state prevails, and consequently precipitation processes occurred and the solution unable to dissolve any quantity of minerals.
INPUT DATA (Chemical Analysis)
Ionic Strength Activity of ions
Activity of complex
Log
Ionic activity product
(IAP)
Solubility product
(KT)
Mol. conc. of complexes
Mol. conc. of species
SATURATION INDEX
(SI)
SOLUBILITY PRODUCT
(KT)
> 0.0
SUPERSATURATION
= 0.0
EQUILIBRIUM
< 0.0
SUBSATURATION
NO. | CONSTRAINTS | PHASES |
1 | Sodium | Na-Mont. |
2 | Potassium | K-Mont. |
3 | Calcium | Ca-Mont. |
4 | Magnesium | Dolomite |
5 | Chloride | Sodium Chloride |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1559
ISSN 2229-5518
5.4.2. Model results:
The
results
obtained from
NETPATH
have been established (Tables 3&4 ) The net geochemical mass-balance reactions between initial and final waters of ten Profiles selected along the groundwater flow path of the Pleistocene aquifer (Fig.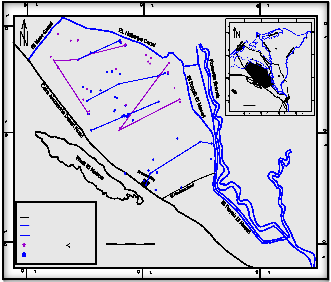
14 ) was preceded, Sodium, Potassium, Calcium, magnesium, Chloride, Sulfur, Carbon, iron, Manganese, Nitrogen and Phosphorus were introduced
30 00
into the model as constraints
. Na-
3
montmorill
onite, K- montmorillonite, Ca-montmorillonite, Dolomite, Sodium Chloride, Gypsum, Aragonite, Goethite, Rhodochr, NH3 gas and Vivianit were selected as interactive phases of the aquifer. The results of mass – balance transfer along groundwater flow path are presented in (Tables 3&4 ) .
30 30 31 00
82
80
81 79
H
21
18
1766
19
6563
6416
31
30
Lake Idku
Mediterranean Sea
Rosetta
Damietta
Kafr El Sheikh
71
7270
7473
2067
C
24
2758
6689
D
36
26
35 34
83
33
G
32
3130
61
60
62
59 55
58
1011
14
84
B
31
00 Abu El Matamir
30
30
The Study Area
Damanhur
Kafr Bolin
El Sadat City
Tanta
Shibin
El Kom
Kafr
Dawoud
El Khatatba
Banha
El Mansoura
77
1
52
9
29
76 28
232725 5
85
51 86
30
00 Km.
0 10 20 30
Cairo
8 3 6 27
7
F 2
30 00
30 30
31 00
31 30
53
37 54
50
49 Initial Point
43
E
40 44
39
48 38 41
47
42
Final Point 45
46
Legand
Main Road
Main irrigation Canal Secondary irrigation Canal Shallow well ( 65 m )
Deep well ( 65 m - 170 m )
0 4 12 20 Km.
Scale
30 00
30 30
31 00
Pleistocene aquifer in the study area.
Table (4): Minerals results of mass balance transfer (NETPATH MODEL) along a flow path in shallow groundwater wells of the Pleistocene aquifer in the study area.
Well No. | Na- Mon | K- Mon | Ca- Mon | Dolomite | Gypsum | Sodium Chloride | Aragonite | Geothite | Rhodochr | Vivianit |
85 & 54 | 6.929 | 0.156 | -2.724 | 0.0421 | 2.247 | 17.638 | -1.232 | -0.011 | 0.0005 | 0.0047 |
55 & 54 | 3.971 | 0.311 | 11.880 | 0.372 | 4.852 | 18.005 | -6.132 | 0.00015 | 0.0015 | 0.0016 |
70 & 75 | 22.01 | 2.099 | -18.29 | 3.360 | 7.933 | 17.816 | -8.355 | -0.0089 | 0.0034 | 0.0040 |
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1560
ISSN 2229-5518
70 & 64 | 23.686 | 2.566 | -15.46 | 2.186 | 9.240 | 19.233 | -7.760 | -0.011 | 0.00124 | 0.0029 |
Well No. | Na- Mon | K- Mon | Ca- Mon | Dolomite | Gypsum | Sodium Chloride | Aragonite | Geothite | Rhodochr | Vivianit |
43 & 42 | 5.285 | 2.797 | 3.564 | 1.0029 | 7.815 | 15.918 | -5.460 | -0.0009 | 0.00115 | 0.00084 |
51& 37 | - 6.925 | 1.979 | 2.582 | 0.540 | -0.0193 | 9.039 | -0.781 | -0.0007 | 0.0018 | 0.00056 |
12 & 1 | - 3.482 | 0.372 | 8.517 | 1.361 | 2.043 | 10.731 | -4.186 | -0.0004 | 0.00184 | 0.00065 |
20 & 18 | - 7.276 | 0.388 | 3.704 | 1.841 | 2.743 | 4.802 | -3.244 | -0.0007 | -0.00151 | -0.0004 |
e results obtained from NETPATH have been established (Tables 3 and 4 ) The net geochemical mass-balance reactions between initial and final waters of ten Profiles selected along the groundwater flow path of the Pleistocene aquifer (Fig. 14 ) was preceded, Sodium, Potassium, Calcium, magnesium, Chloride, Sulfur, Carbon, iron, Manganese, Nitrogen and Phosphorus were introduced into the model as constraints. Na-montmorillonite, K-montmorillonite, Ca-montmorillonite, Dolomite, Sodium Chloride, Gypsum, Aragonite, Goethite, Rhodochr, NH3 gas and Vivianit were selected as interactive phases of the aquifer.
The following can be concluded:
a) Profile (A): The results of mass–balance transfer along this profile reflect precipitation of -2.724 mmole of Ca-mon, -1.232 mmole Aragonite, -0.011 mmole Goethite and dissolution of 6.929 mmole Na-mon, 0.156 mmole K- mon, 0.0421 mmole Dolomite,
2.247 mmole Gypsum, 17.638 mmole Sodium
Chloride, 0.0005 mmole Rhodochr and
0.0047 mmole Vivianit (all expressed mmole per kg of groundwater).
b) Profile (B): The mass–balance transfer along this profile reflect precipitation of -6.132 mmole aragonite and dissolution of 3.971mmole Na- Mont,
0.311mmole K- Mont, 11.880 mmole Ca- Mont, 0.372
mmole Dolomite, 4.852 mmole Gypsum, 18.005 mmole Sodium Chloride, 0.00015 mmole Goethite,
0.0015 mmole Rhodochr, 0.0016 mmole Vivianit.
c) Profile (C): The mass–balance transfer along this profile precipitation -18.29 mmole of Ca- Mont, -
8.355 mmole of Aragonite, -0.0089 mmole of
Goethite and dissolution of 22.01 mmole Na-Mon,
2.099 mmole K-Mon, 3.360 mmole Dolomite, 7.933 mmole Gypsum, 17.816 mmole Sodium Chloride,
0.0034 mmole Rhodochr, 0.0040 mmole Vivianit.
d) Profile (D): The mass–balance transfer along this profile reflect precipitation of -15.46 mmole of Ca- Mont, -7.760 mmole of Aragonite, -0.011mmole of Goethite and dissolution of 23.686 mmole Na-Mon,
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1561
ISSN 2229-5518
2.566 mmole K-Mon, 2.186 mmole Dolomite, 9.240 mmole Gypsum, 19.233 mmole Sodium Chloride,
0.00124 mmole Rhodochr, 0.0029 mmole Vivianit.
e) Profile(E): The results of mass–balance transfer along this profile reflect precipitation of -5.460 mmole Aragonite, -0.0009 mmole Goethite and dissolution of
5.285 mmole Na-Mon, 2.797 mmole K-Mon, 3.564
mmole Ca-Mon, 1.0029 mmole Dolomite, 7.815 mmole Gypsum, 15.918 mmole Sodium Chloride,
0.00115 mmole Rhodochr and 0.00084 mmole
Vivianit.
f) Profile (F): The results of mass–balance transfer along this profile reflect precipitation of -6.925 mmole Na-Mon, -0.0193 mmole Gypsum, -0.781 mmole Aragonite, -0.0007 mmole Goethite and dissolution of
1.979 mmole K-Mon, 2.582 mmole Ca-Mon, 0.540 mmole Dolomite, 9.039 mmole Sodium Chloride,
0.0018 mmole Rhodochr and 0.00056 mmole Vivianit. g) Profile (G): The results of mass–balance transfer along this profile reflect precipitation of -3.482 mmole Na-Mon, -4.186 mmole Aragonite, -0.0004 mmole Goethite and dissolution of 0.372 mmole K-Mon,
8.517 mmole Ca-Mon, 1.361 mmole Dolomite, 2.043 mmole Gypsum, 10.731 mmole Sodium Chloride,
0.00184 mmole Rhodochr and 0.00065 mmole
Vivianit.
h) Profile (H): The results of mass–balance transfer along this profile reflect precipitation of -7.276 mmole Na-Mon, -3.244 mmole Aragonite, -0.0007 mmole Goethite, -0.00151 mmole Rhodochr, -0.0004 mmole Vivianit and dissolution of 0.388 mmole K- Mon, 3.704 mmole Ca-Mon, 1.841 mmole Dolomite,
2.743 mmole Gypsum and 4.802 mmole Sodium
Chloride.
The predominant reactions for all profiles appear to be dissolution of Sodium Chloride, Gypsum, Dolomite, Rhodochr and Vivianit ., and precipitation
of of Ca-mon, Aragonite, Goethite – with different values . So there is increase in salinity in all profile in the same direction of groundwater flow, also dissolution of both Rhodochr and Vivianit leads to increase the concentration of nitrates and phosphate as a result of extensive use of chemical fertilizer and pesticides .
Even through primary silicate minerals (clay minerals) predominate in the mineralogy of the Quaternary aquifer in the study area, the mass balance modeling indicates that reactions with these minerals are secondary to those of the more reaction carbonate , sulfate and chlorides and precipitation of Ca-mon, K- mon , and Na-mon. in different profiles. [16] and [17] .
This reaction agreement with the sequence of
cations (Na+>Ca++ > Mg++), (Na+>Mg++ > Ca++) and anions (Cl- > SO4 — >HCO3- ). From these profiles we notes the groundwater change from less advanced stage of mineralization (HCO3- > SO4 — > Cl-) to more advanced stage of mineralization (Cl- > SO4 —
>HCO3-)
Groundwater of the Pleistocene aquifer in the
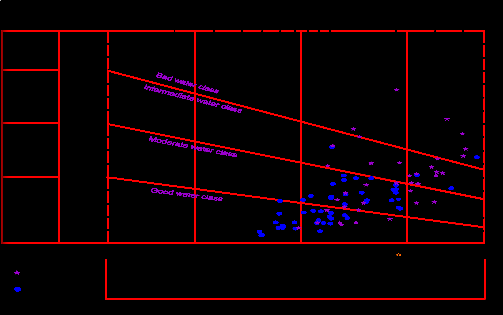
study area is evaluated according to [18] based on the Sodium Adsorption Ratio (SAR), and the specific conductance (Fig. 15). The U.S. Salinity Laboratory diagram is divided into four classes C1, C2, C3 and C4, which denote the conductance and S1, S2, S3 and S4 which denote the sodium adsorption ratio (SAR). The recommended classification of water for irrigation according SAR ranges are shown in (Table).
SAR = Na+/√ (ca++ + Mg++) /2
Where, the concentrations of these cations are expressed
in mq/l according to the U.S. Salinity Laboratory diagram.
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1562
ISSN 2229-5518
Sodium
(alkali)
Hazard
Very
High
100 500 1000
30
5000
30
S4
High
S3
Medium
S2
Low
S1
28
26
24
22
20
18
16
14
12
10
8
6
4
2
100
C1-S4
C1-S3
C1-S2
C1-S1
C2-S4
C2-S3
C2-S2
C2-S1
250
C3-S4
C3-S3
C3-S2
C3-S1
750
C4-S4
20
C4-S3
10
C4-S2
C4-S1
2250 0
Shallow well
Deep well
Conductivity (micromhos/cm at 25 C)
Salinity Hazard
(Fig. 15): Water classification for irrigation according to US Salinity Lab. 1954) [19]
As shown from figure 15, it clears that: most of groundwater samples are good to moderate water class and suitable for irrigation purposes and only 17 % of shallow groundwater samples and 2 % of deep groundwater samples falls in the bad water classes (C3- S4) and (C4-S4) which are unsatisfactory.
Trace elements:
Iron content was measured in the collected groundwater samples, it ranging. ranging from 0.04 mg/l to 0.36 mg/l in shallow production wells, and ranging from 0.01 mg/l to 0.18 mg/l in the majority of groundwater samples in deep production wells . Iron concentration increases gradually toward the southeastern portions. According to the standard acceptable limits (0.3 mg/l) [19] and [20], , Egyptian standard for drinking and domestic uses all samples of deep groundwater wells are suitable value of iron according to the same standard limits. also most of shallow groundwater wells are suitable value of iron according to the same standard limits . Except 32.5 % of recorded samples polluted with iron according to the same standard limits .
0.06 mg/l to 0.32 mg/l in deep production wells in the study area. The recommended maximum of manganese concentration for public water supplies is 0.05 mg/l [19]. The concentration of manganese content according to the Egyptian standard limits (0.1 mg/l), most groundwater samples in the study area is polluted
with manganese. Which reflects effect of dissolution of
manganese bearing deposits?
Phosphate contents in the collected groundwater samples are ranging from 0.02 mg/l to 0.1 mg/l of the majority of groundwater samples . Generally, phosphates enter the water supply from agricultural fertilizer run-off, water treatment, and biological wastes and residues.
Results of chemical analysis show that nitrite is not detected in all samples, while concentration of nitrate in studied area ranges from 6 mg/l to 33 mg/l in the major shallow groundwater samples and from 1mg/l to 6.8 mg/l in the major deep groundwater samples high values of Phosphate and Nitrite content related to excessive uses of chemical fertilizers and pesticide .
Conclusion
There is great diversity in environmental impacts in the study area, these effects represented in the effects of agricultural activity and increased reclamation land projects, an addition to activity associated with the different interactions of salts which leads to of dissolution and precipitation of minerals. These environmental impacts lead to affects on the quality of water, and also on the evolution of water and these appear through the results of the program (Netpath).
Using geochemistry as a tracer of groundwater flow requires a clear understanding of
the origin of the geochemical patterns, in line with
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1563
ISSN 2229-5518
the suggestion by [21], [22] and [15] and Netputh program. There is a clear geochemical zonation in the composition of the study area waters following the geological sequence. This zonation is characterized by a change of cation species from dominantly Ca and Mg near the east to Na- dominated waters in the west. Mirroring this, anions change from HCO3 type to Cl and SO4 type.
So, The consequent of cations and anions change, the
Water was evaluated for irrigation using SAR
sodium adsorption ratio, the majority of groundwater samples in the study area are suitable for irrigation.
1- Gallardo, A. H., and Tase, N. (2007): Hydrogeology and geochemical characterization of groundwater in a typical small-scale agricultural area of Japan. Journal of Asian Earth Sciences, 29(1), 18-28. [doi:10.1016/j.jseaes.2005.12.005]
2- Xu, Z. H., Li, Y. F., Jiang, L., Hou, G. C., and Hu, A. Y. (2009): Geochemical modeling of Huanhe water-bearing layers in South Ordos Basin. Journal of Arid Land Resources and Environment, 23(9), 160-168. (In Chinese).
3- Wang, P. M., Anderko, A., Springer, R. D., Kosinski, J. J., and Lencka, M. M. (2010): Modeling chemical and phase equilibria in geochemical systems using a speciation-based model. Journal of Geochemical Exploration,
106(1-3), 219-225. [doi:10.1016/j.gexplo.2009.09.003]
4- Belkhiri L, Boudoukha A, Mouni L, Baouz T. (2010): Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater - A case study: Ain Azel plain (Algeria). Geoderma159:390–398.
5- ASTM, American Society for testing and material (2002). In "water and environmental technology". annual book of ASYM standars, Sec. 11, Vol. 11.01 and 11.02, West Conshohocken, U.S.A.
6- Plummer NP, Prestemon EC, Parkhurst DL.(1994). An interactive code NETPATH for modelling net geochemical reactions along a flow path_version 2.0. United States Geological Survey, 120 p.
7- Lyon WB, Bird DA. (1995): Geochemistry of the Madeira River Brazil comparison of seasonal weathering reactions using a mass balance approach. J S Am Earth Sci ;8(1).:97-101
8- Soulsby C, Chen M, Ferrier RC, Helliwell RC, Jenkins R, Harriman R.(1998). Hydrogeochemistry of shallow
groundwater in an upland Scottish catchment. Hydrol Process;12: 1111-1117.
9- El Abd, A.E. (2005): "The geological impact on the water bearing formations on the area southwest Nile Delta, Egypt". Ph.D. Thesis, Fac. Sci. Menofiya Universuty.
10- Starinsky, A.; Bielski, M.;Eckerr, A. and Steinitz, G.(1983). Tracing the origin of salts in groundwater by Sr isotopic composition (The crystalline complex of the southern Sinai, Egypt), Isotope Geoscience, 1: 257-267.
11- Todd, D. K. (1959). Groundwater Hydrology 2 nd edition., John Wiley and Sons Inc., New York, U.S.A., 336
12- Piper , A.M.(1953 ): "Agraphic representation in the Geochemical interpretation of groundwater analysis" Am.
Geophis. Union Transactions, 25:914-923.
13- Truesdell, A. H., and Jones, B. F., (1974), WATEQ, a computer program for calculating chemical equilibria of natural waters: Journal of Research, U.S. Geological Survey, v. 2, p. 233-274.
14- Plummer, L.N. Parhurst, D.L; Fleming, G.W. and Dunkel, S.A. (1988): A computer program incorporating pitzers equations for calculation of geochemical reactions in brines. U.S. Geol. Surv., Water Resour. Inv. Rep. P.
88-4153.
15- Glynn PD, Plummer LN. (2005) Geochemistry and understanding of groundwater systems. Hydrogeology J. volume 13: pp 263–287.
16- Appelo CAJ and Postma D. (2005). Geochemistry, groundwater and pollution.A.A. ,second edition, Amesterdam, the Netherlands, Balkema 649 p.
17- Postma
IJSER © 2015 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 6, Issue 4, April-2015 1564
ISSN 2229-5518
18- Salinity Laboratory: ( 1954) ; "Diagnoses And Improvements of Saline And Alkaline Soil "S. S. Dept. of
Agriculture , Hand Book , No. 60 ,pp. 160.
19- World Health Organization (WHO) (2005). International standards for drinking waters. 5th Edition, Vol. 1
Geneva, Switzerland
20- Environmental Protection Agency (E .P .A .); ( 1984 ): " National Intrim. Primary Drinking Water Regulation " U.S. E P A Part 141, Federal Register 40 (248 ), Dece.24 , pp. 59566 – 59588.
21- Domenico, P.A., and Schwartz, F.W.,(1990), Physical and chemical hydrogeology: John Wiley and Sons, New
York, 824 p.
22- Hem, J. D., (1989): Study and interpretation of the chemical characteristic of natural water. U.S. geol. Surv., Water supply paper 2254, 3rd edition, 264 p.
23- Burdon, D.J. (1958). Metasomatism of groundwater at depth, UNESCO course on Hydrology, Desert Institute, Cairo, Egypt
24- Back, W. and Hanshaw, B. B. (1979): “Major geochemical processes in the evolution of carbonate aquifer
system’’. Elsevier Scientific Publishing Company, Amsterdam, the Netherlands.
IJSER © 2015 http://www.ijser.org