International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1369
ISSN 2229-5518
Adiponectin is a macromolecular complex similar to the members of the C1q and other collagenous homologues. Adiponectin is secreted by white adipose tissue and as known as for its function it is anti-diabetes, anti-atherosclerosis, anti-inflammation and antitumor activities, which have been directly linked to the high molecular weight (HMW). Oligomeric structures formed by multiples of Adiponectin trimmers. Adiponectin receptors in various organs and tissues clearly show that adiponectin has pleiotropic effect on numerous physiological process. Evidence suggests that adiponectin may also have anticancer Properties, cardio protective and effect on female reproductive. Adiponectin has two receptor .The two receptors for Adiponectin, AdipoR1 and AdipoR2 have been characterized that mediate effects of Adiponectin in various tissues. These receptors contain 7- transmembrane domains but are structurally and functionally distinct from G-protein-coupled receptors. In this study we will shows the characterization and properties of Adiponectin, AdipoR1 and AdipoR2 and their relation with obesity and cardiovascular disease and type 2 diabetes . Keyword: Adiponectin, AdipoR1 receptor, AdipoR2 receptor, Obesity, diabetes.
Adipocytes are the cells that primarily compose adipose tissue, specialized in storing energy as fat. There are two types of adipocytes, brown and white, which differ in several important properties. White adipose tissue is important for maintaining energy metabolism of the organism by storing excess energy as lipid. Adipose tissue is made up from a variety of different cells.
The most prominent fraction are mature adipocytes which store and release lipids in response to circulating hormones. White adipocyte cells from different locations can have distinct molecular and physiological properties such as increased visceral adipose tissue is associated with an increased risk of insulin resistance and cardiovascular disease, Furthermore adipocyte size has been linked to an increased risk of metabolic complications such as Type 2 diabetes or cardiovascular disorders.
Recent research has shown that adipose tissue is not simply an inert storage depot for lipids but is also an important endocrine organ that plays a key role in the integration of endocrine, metabolic, and inflammatory signals for the control of energy homeostasis. The adipocyte has been shown to secrete a variety of bioactive proteins into the circulation. These secretory proteins, which have been collectively named adipocytokines , include leptin , tumor necrosis factor (TNF)-α , plasminogen-activator inhibitor type 1 (PAI-1) , adipsin , resistin and adiponectin(Manju et al., 2003).
Adiponectin was independently discovered between 1995 and 1996 and given different names including apM1(adipose most abundant gene transcript 1) (Maeda et al.,1996), Acrp30 (adipocyte complement-related protein of
30 kDa) (Scherer et al., 1995), GBP28 (gelatin binding
protein of 28 kDa) (Nakano et al., 1996) and adipQ (Hu et
al., 1996).
Adiponectin is a protein hormone that modulates a number of metabolic processes, including glucose regulation and fatty acid catabolism and secreted from adipose tissue into the bloodstream and is very abundant in plasma relative to many hormones(Takashi and Toshimasa,
2005). Adiponectin has beneficial effects on obesity-related medical complications. In contrast to other adipokines, circulating adiponectin levels are reduced in obesity, type 2 diabetes and associated diseases(Li et al., 2009).
Adiponectin or AdipoQ, apM1 or GBP28 is a cytokine produced exclusively and is abundant in human plasma with concentrations of 5–30 (μg/mL), thus accounting for approximately 0.01% of total plasma proteins. The levels of adiponectin are reduced in diabetics compared to non- diabetics(Anthony et al., 2006).
As the definition of diabetes in The World Health Organization, Diabetes is a chronic disease, which occurs when the pancreas does not produce enough insulin or when the body cannot effectively use the insulin produces(Chung et al., 2012). This leads to an increased concentration of glucose in the blood (hyperglycemia)( Xuhua et al., 2012). There are three main forms of diabetes mellitus: type 1, type 2, and gestational diabetes (occurring during pregnancy), which have different causes and population distributions(Chung et al., 2012). According to International Diabetes Federation (IDF) figures released in
2006, the disease diabetes now affects a staggering 246
million people worldwide, with 46% of all those affected in
the 40-59 age group. Several studies showed that low levels of adiponectin can be assigned to human diseases which has recently been summarized by Díez and Iglesias (2003). Reduced levels of adiponectin are associated with obesity, hypertension and type 2 diabetes mellitus(Xuhua et al.,
2012 and Chung et al., 2012). Although the precise physiological role of adiponectin is not yet fully
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1370
ISSN 2229-5518
understood, its function as an anti-inflammatory and anti- atherogenic compound is well established( Villarreal and Antuna, 2012). Also increased adiponectin levels are associated with increased insulin sensitivity and glucose tolerance. Thus, adiponectin might be useful for therapeutic application against diseases associated with insulin resistance such as type 2 diabetes mellitus and obesity. Because of its anti-inflammatory effect it might be useful as a protective factor for atherosclerosis development in cases where adiponectin levels in plasma are low(Xuhua et al.,
2012 and Chung et al., 2012 and Villarreal and Antuna,
2012). Furthermore, increasing plasma adiponectin might
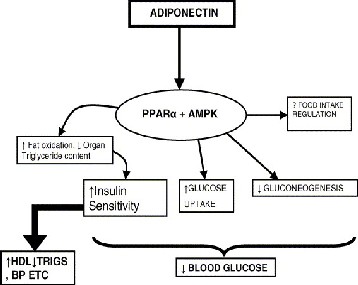
also be useful in preventing vascular restenosis after vascular intervention. Circulating levels of adiponectin are decreased in obesity , dyslipidemia, cardiovascular disease (CVD) , insulin resistance and T2DM. Adiponectin enhances AMP-activated protein kinase activity in the arcuate hypothalamus via its receptor AdipoR1 to stimulate food intake and decreases energy expenditure (Susanne et al., 2007 and Clinton et al., 2013).
More than 70% of patients with type 2 diabetes die of
cardiovascular causes. Low circulating Adiponectin levels
have been associated with cardiovascular disease (CVD), which are increased risk of acute coronary syndrome, progression of coronary calcification, the extent of CVD and coronary lesion complexity and heart rate-corrected QT interval( Villarreal and Antuna, 2012 and Markku, 2008). Adiponectin is synthesized and secreted by human cardiomyocytes which are expressed AdipoR1 and AdipoR2. In vitro studies suggesting that Adiponectin may also protect cardiomyocytes in a direct manner, in addition to the indirect protection provided by its vascular effects(Xuhua et al., 2012 and Anastasios et al., 2011).
Adiponectin cDNA was first isolated by large scale random sequencing of the human adipose tissue cDNA library. It is a collagen-like protein that is exclusively synthesized in white adipose tissue, and is induced during adipocyte differentiation and circulates at relatively high (μg/mL) concentrations in the serum. Once synthesized, mammalian adiponectin undergoes posttranslational hydroxylation and glycosylation yielding eight isoforms. Six of the adiponectin isoforms are glycosylated(VA Kothiwale et al., 2010).
Adiponectin is a protein of 247 amino acids consisting of four domains, an amino-terminal signal sequence, a variable region, a collagenous domain (cAd), and a carboxy-terminal globular domain (gAd)( Scherer et al.,
1995). The basis of both its primary amino acid sequence and its subunit domain structure. Adiponectin is most
similar to C1q, which is a member of the complement- related family of proteins(Berg et al., 2002).
The basic building block of adiponectin is a tightly associated trimer, which is formed by association between three monomers at the globular domains. Monomeric (30- kDa) adiponectin has not been observed in the circulation and appears to be confined to the adipocyte. Four to six trimers associate through their collagenous domains to form higher-order structures or oligomers, which circulate in plasma(Scherer et al., 1995 and Berg et al., 2002 and Arita et al., 1999).
Adiponectin has been postulated to play an important role in the modulation of glucose and lipid metabolism in insulin-sensitive tissues in both humans and animals(Yamauchi et al., 2001). Decreased circulating adiponectin levels have been demonstrated in genetic and diet-induced murine models of obesity, as well as in diet- induced forms of human obesity(Arita et al., 1999). Low adiponectin levels have also been strongly implicated in the development of insulin resistance in mouse models of both obesity and lipoatrophy (Yamauchi et al., 2001). In humans, plasma levels of adiponectin are significantly lower in insulin-resistant states including type 2 diabetes and can be increased upon administration of the insulin-sensitizing thiazolidinedione (TZD) class of compounds (Weyer et al.,
2001 and Maeda et al., 2001 and Combs et al., 2002).
Plasma adiponectin levels in diabetic subjects with coronary artery disease (CAD) are lower than in diabetic patients without CAD, which are suggesting that adiponectin may have anti-atherogenic properties (Hotta et al., 2000). Adiponectin has been shown to dose- dependently decrease the surface expression of vascular adhesion molecules known to modulate endothelial inflammatory responses (Ouchi et al., 1999). It also inhibits proliferation of vascular smooth muscle cells and concentrates within the vascular intima of catheter-injured vessels (Arita et al., 2002 and Okamoto et al., 2000). The association of low adiponectin levels with obesity, insulin resistance, CAD, and dyslipidemia indicates that this protein can be an important marker of the metabolic syndrome.
The mechanisms of adiponectin are largely unknown. Adiponectin administration has been shown to increase insulin induced tyrosine phosphorylation of the insulin receptor in skeletal muscle in association with increased whole-body insulin sensitivity(Yamauchi et al., 2001). Stimulation of glucose utilization and fatty acid oxidation
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1371
ISSN 2229-5518
in skeletal muscle and liver by adiponectin may also occur through activation of 5′-AMP kinase. 5′-AMP-activated protein kinase play a crucial role in the regulation of energy expenditure and glucose and lipid metabolism. The tissue- specific effect of adiponectin on 5′-AMP kinase has recently been demonstrated in mice. In skeletal muscle of mice, adiponectin showns to increase expression of the genes encoding proteins involved in fatty acid transport and oxidation, such as CD36, acyl-CoA oxidase and uncoupling protein, which are resulting in enhanced fat combustion and energy dissipation(Yamauchi et al., 2002). In the liver, low doses of adiponectin decreased the expression of proteins involved in fatty acid transport, such as CD36, leading to reduced fatty acid influx into the liver and hepatic triglyceride content(Yamauchi et al., 2003) . Although adiponectin is secreted only from adipose tissue, its levels are paradoxically lower in obese than in lean humans. This is in contrast to most other adipocytokines, whose levels are increased in obesity in proportion to an increased total body fat mass. It is possible that although adiponectin expression is activated during adipogenesis, a feedback inhibition on its production may occur during the development of obesity(Kappes and Loffler , 2000). Levels are also lower in diabetic patients compared with nondiabetic subjects and are particularly low in subjects with CAD. Decreased levels are found in men compared with women and this may be androgen induced(Valsamakis et al., 2003).
Two receptors for adiponectin are AdipoR1 and AdipoR2, which have been characterized that mediate effects of adiponectin in various tissues. These receptors contain 7-
transmembrane domains but are structurally and functionally distinct from G-protein-coupled receptors, displaying intra-cellular and extracellular N- and C-termini and signaling via alternate, non-classic GPCR path- ways(Hayley et al., 2010 and Sahar et al., 2013).
AdipoR1 is expressed ubiquitously, most abundantly in
skeletal muscle, whereas AdipoR2 is predominantly
expressed in the liver. AdipoR1 functions as a high-affinity receptor for globular adiponectin and a low-affinity one for full-length adiponectin(Hyun et al., 2010).
adiponectin suppresses SREBP1c by AdipoR1, one of the
functional receptors for adiponectin, and furthermore that
suppressing either AMP-activated protein kinase (AMPK) via its upstream kinase LKB1 deletion cancels the negative effect of adiponectin on SREBP1c expression(Motoharu et al., 2009).
Endoplasmic reticulum protein 46 (ERp46) interacts
specifically with AdipoR1 and provide evidence that ERp46 modulates adiponectin signaling, only AdipoR1 is constitutively expressed on the cell-surface(Hayley et al.,
2010).
Insulin repressed the mRNA expression of AdipoR1 and
AdipoR2 via activation of PI3-kinase and inactivation of Foxo1. LXR agonists increased the expression of AdipoR1and AdipoR2 in human macrophages, whereas agonists of PPARa and PPARc only increased the expression of AdipoR2(Takashi et al., 2007).
AdipoR1 increases AMPK activation by adiponectin in liver. Activation of AMPK in the liver has been reported to reduce the expression of genes encoding hepatic gluconeogenic enzymes such as glucose-6-phosphatase (G6pc) and phosphoenolpyruvate carboxykinase 1 (Pck1) as well as genes encoding molecules involved in lipogenesis such as sterol regulatory element binding protein 1c (Srebf1) . In fact, expression of AdipoR1 significantly decreased the expressions of G6pc, Pck1 and Srebf1 in the liver of db/db mice, which may be among mechanisms by which restoration of AdipoR1 in the liver reduced endogenous glucose production (EGP), apparently increased glucose infusion rate (GIR) and improved diabetes. These results suggested that AdipoR1 may be more involved in the activation of AMPK by adiponectin than AdipoR2 in liver in vivo(Motoharu et al., 2009).
In humans, AdipoR2 is correlates positively with insulin
sensitivity and fasting plasma triglyceride concentrations in healthy glucose tolerant subjects. Endoplasmic reticulum(ER) stress or obesity-inducible ATF3 negatively regulates the expression of adiponectin and AdipoR2(Takashi et al., 2007). On the contrary, the expression level of AdipoR2, but not AdipoR1 was decreased in the intra-abdominal AT of obese individuals and correlated negatively with triglyceride and apolipoprotein B levels. The non-conserved region of
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1372
ISSN 2229-5518
AdipoR2 (residues 1–81) restricts its cell-surface expression (Sahar et al., 2013). Introduction or deletion of this region, into AdipoR1 or AdipoR2 resulted in inhibition or promotion of cell-surface expression. AdipoR2 acts as an intermediate-affinity receptor for both globular and full- length adiponectin. Expression of AdipoR2 had little effects on the expression levels of G6pc, Pck1 or Srebf1(Motoharu et al., 2009).
Expression of AdipoR2 significantly increased the
expression of genes encoding molecules involved in glucose uptake such as glucokinase (Gck) and unlike the molecules involved in gluconeogenesis, which appeared to be one possible mechanism by AdipoR2 expression in the liver apparently increased GIR and improved diabetes (John et al., 2009).
In conclusion we find that the adipocyte as simply a storage depot for fat is no longer tenable. Among, Adiponectin, which is solely in adipose tissue, appears to play a very important role in lipid and carbohydrate metabolism and vascular biology. Adiponectin appears to be a major modulator of insulin action and its levels are reduced in type 2 diabetes, which could contribute to peripheral insulin resistance in this condition. It has significant insulin-sensitizing as well as anti-inflammatory properties that include suppression of macrophage phagocytosis and blockage of monocyte adhesion to endothelial cells in vitro. Although further investigations are required, adiponectin administration, as well as regulation of the pathways controlling its production, represents a promising target for managing obesity, hyperlipidemia, insulin resistance, type
2 diabetes, and vascular inflammation.
Anastasios K, Panayoula CT, Ignatios I, Eirini M, Panayota M, Erifyli K, Eleni B, John L, Theofanis E, Dimitrios TK, George D, Sotirios AR. 2011. Adiponectin levels and expression of adiponectin receptors in isolated monocytes from overweight patients with coronary artery disease. Cardiovascular Diabetology. 10 : 14–26.
Anthony EC, Barbara U, Stacy C, Matthew H, Ralph A, DeFronzo, Lawrence M, Eric R and Steve RS. 2006. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metabolism. 4: 75–87.
Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. 2002. Adipocyte-derived
plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common post receptor signal in vascular smooth muscle cell. Circulation. 105 : 2893–2898.
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. 1999. Paradoxical decrease of an adipose- specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 257 : 79–83.
Berg AH, Combs TP, Scherer PE, 2002. Acrp30/adiponectin: an adipocytokine regulating glucose and lipid metabolism. Trends Endocrinol Metabolism. 13 : 84–89.
Christa B, Josef W, Markus N. 2010. Adiponectin receptor binding proteins – recent advances in elucidating, adiponectin signalling pathways. FEBS Letters. 584(20):
4280–6.
Chung HH, Ying LL, Su CL and Pesus C. 2012. Adiponectin Level Predicts HDL-Cholesterol Level in Type 2 Diabetes. The Open Atherosclerosis & Thrombosis Journal. 5 : 1–5.
Clinton MH , Eric DB , David HW. 2013. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Molecular and Cellular Endocrinology.
366(2) : 152–162.
Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O’Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. 2002. Induction of adipocyte complement related protein of 30 kilodaltons by PPAR-gamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 143 : 998–1007.
Hayley KC, Julie W, Sarah K, Fiona S, Ayanthi AR, Jonathan PW. 2010. ERp46 binds to AdipoR1, but not AdipoR2, and modulates adiponectin signaling. Biochemical and Biophysical Research Communications. 392(2) : 234-239.
Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. 2000. Plasma concentration of a novel adipose specific protein adiponectin in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 20 : 1595–1599.
Hyun JP, Young MK, Cho HK, Myeong HJ. 2010. ATF3 negatively regulates adiponectin receptor 1 expression.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1373
ISSN 2229-5518
Biochemical and Biophysical Research Communications. 400 : 72-
7.
John TH, Cornelia MW, Cathleen J, David K, Karin M, Annette GB. 2009. Protein kinase CK2 interacts with adiponectin receptor 1 and participates in adiponectin signaling. Cell signal. 21 : 936-42.
Kappes A, Loffler G. 2000. Influences of ionomycin, dibutyryl-cycloAMP and tumor necrosis factor alpha on intracellular amount and secretion of apM1 in differentiating primary human preadipocytes. Hormone Metab Res. 32 : 548–554.
Li S, Shin HJ, Ding EL, van DRM. 2009. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta- analysis. JAMA. 302 : 179-88.
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. 2001. PPAR gamma ligands increase expression and plasma concentrations of adiponectin an adipose derived protein. Diabetes. 50 : 2094–2099.
Markku L. 2008. Cardiovascular Disease in Type 2 Diabetes
From Population to Man to Mechanisms. Diabetes Care. 33 :
2442-49.
Matsubara M, Maruoka S, Katayose S. 2002. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 87 : 2764–2769.
Motoharu A, Kohjiro U, Kazunori I, Toshimasa Y, Kazuma K, Yukiko O, Nabeel B, Shin O, Ryozo N, Takashi K. 2009. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 382(1):51-6.
Okamoto Y, Arita Y, Nishida M. 2000. An adipocyte- derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 32 : 47–50.
Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y, 1999. Novel modulator for endothelial adhesion molecules: adipocyte- derived plasma protein adiponectin. Circulation. 100 : 2473–
2476.
Sahar K, Felicity JR, Hayley KC, Nicole LS, Julie W, Yu HK, Choaping N, Robert GP, Jonathan PW. 2013. Characterisation of the adiponectin receptors: The non- conserved N-terminal region of AdipoR2 prevents its expression at the cell-surface. Biochem Biophys Res Commun. 432(1):28-33.
Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF.
1995. A novel serum protein similar to C1q produced
exclusively in adipocytes. J Biol Chem. 270 : 26746–26749.
Susanne R, Anne CA, Ling L, Bernd N. 2007. Age- associated loss in adiponectin-activation by caloric restriction: Lack of compensation by enhanced inducibility of adiponectin paralogs CTRP2 and CTRP7. Mol Cell Endocrinol. 277(1-2):26-34.
Takashi K, Toshimasa Y, Naoto K. 2007. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 582(1):74-80.
Takashi K and Toshimasa Y. 2011. Adiponectin Receptor Signaling: A New Layer to the Current Model. Cell metabolism. 13(2) : 123–124.
Takashi K and Toshimasa Y. 2005. Adiponectin and
Adiponectin Receptors. Endocr Rev. 26(3):439-51.
Valsamakis G, Chetty R, McTernan PG, Al-Daghri NM, Barnett AH, Kumar S. 2003. Fasting serum adiponectin concentration is reduced in Indo- Asian subjects and is related to HDL cholesterol. Diabetes Obes Metab. 5 : 131–5.
VA Kothiwale, Madhav p, Belgaum. 2010. Adiponectin-the molecule of this decade. Medicine. 20 : 172-177.
Villarreal M, Antuna P. 2012. Adiponectin: Anti- inflammatory and cardioprotective effects. Biochimie. 94(10):2143-9.
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. 2001. Hypoadiponectimia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 86
: 1930–1935.
Xuhua S, Hongwei L, Weiping L, Xing W, Xiaosong D.
2012. Pioglitazone prevents hyperglycemia induced
decrease of AdipoR1 and AdipoR2 in coronary arteries and
coronary VSMCs. Mol Cell Endocrinol. 363(1-2):27-35.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 11, November-2013 1374
ISSN 2229-5518
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida
S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. 2002. Adiponectin stimulates glucose utilization and fatty acid oxidation by activating AMP-activated protein kinase. Nat Med. 8 : 1288–1295.
Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. 2003. Globular adiponectin protected ob/ob mice from diabetes and ApoE- deficient mice from atherosclerosis. J Biol Chem. 278 : 2461–
2468.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. 2001. The fat derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 7 :
941–946.
Yizhou X, Ningfu W, Feng L, Peizhang L, Yan G. 2009. Receptor for activated C-kinase 1, a novel binding partner of adiponectin receptor 1. Biochem Biophys Res Commun. 378(1):95-8.
• Co-Author name is Ph.D Researcher Student, Gauhati University , India, PH-03612700311. E-mail: Morteza.Kordafshari@gmail.com .
IJSER © 2013 http://www.ijser.org