
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 734
ISSN 2229-5518
Abstract:
NANDINI.N* and SIVASAKTHIVEL.S
The main intention of this study to understand the microbial consortium of Sulphate Reducing Bacteria(SRB) and Sulphure oxidizing bacteria (SOB) on Extra polymeric substance produced by Pseudomonas sp., with combination of E-coli. In stressing the importance of anaerobic corrosion by SRB, cor- rosive effects of acids produced by microorganisms may be overlooked Sulphur oxidizers chiefly of the genus Thiobacillus sp., which is a strongly corro- sive agent to be studied to understand their activity of SOB and SRB in Microbial Influenced Corrosion. The activity of Sulphate reducing bacteria and Sulphate oxidizing bacteria on biofilm produced by two different combinations of extra polymeric substance (EPS) excreting organisms are discussed in this paper. Microbial Conditioning of Galvanised iron pipes and Remnants Samples (1.5cm x 1.5cm) prepared and introduced in to different sets of aquatic environment. EPS were estimated quantitatively by Micheal Duboisis and Lowery modified method. The activities of SRB and SOB measured by estimating the EPS, before inoculation and after inoculation of SRB and SOB.The result of EPS estimation exposed that the Desulfovibrio sp.,(SRB) enhance the efficiency of Pseudomonas aeruginosa and E-Coli for EPS secretion. After the inoculation of Thiobacillus thioparus(SRB) the slope of EPS step downed and this organisms populated in every in a week of experimental course. The sulphur reducing organism leachate the EPS, or may be consumed for further colonization. The comparative study of Pseudomonas fluorescence and Pseudomonas aeruginosa SEM analysis revealed that Pseudomonas fluorescence secrets the more EPS than Pseudomonas aeruginosa along with combination of E-Coli. The SRB’s are enhances the excre- tion of polymers (Glycogalyx) along with Biofilm forming bacteria by inducing symbiotic relations. The significant of this study is revealed the role of SRB and SOB with association with biofilm forming bacteria in microbial induced corrosion. SRB ‘s enhances the EPS secretion in massive level and SOB’s are consuming the EPS as a energy source for the production acid base products lead to metal corrosion in aquatic environment.
.
Key words— Microbial consortium, SOB, SRB,Bio film,SEM, Pseudomonas fluorescence, Pseudomonas aeruginosa, Polysaccharide, Protein.
—————————— ——————————
Corrosion associated with microorganisms has been recog- nized for over 50 years and yet the study of microbiologically influenced corrosion (MIC) is relatively new. Microbial influ- enced corrosion (MIC) increasingly draws attention from cor- rosion engineers, environmental scientists, and applied micro- biologists, the variety of techniques applied for detection and monitoring also increases. Many microorganisms, principally bacteria, can play a part in corrosion processes, the chief cul- prits are the sulphate-reducing bacteria and sulphur Oxidizing bacteria. Microorganism in nature encounters a wide range of solid surface that may markedly alter their physiological and ecological behavior. A Biofilm is an assemblage of surface- associated microbial cells that enclosed in an Extra cellular polymeric substance matrix [1]. Some microorganism can ad- here directly to the pipe surface via appendages that extend from the cell membrane; other bacteria from a capsular mate- rial of extra cellular polysaccharides (EPS), sometime called a glycocalyx, which anchors the bacteria to the pipe surface [2]. Pseudomonas aeruginosa and E-Coli are primarily colonizing bacteria produce extra polymeric substance on any surface area in the aquatic environment (Natasha ett al; Nandini ett al.,). The majority of Microbially Induced corrosion (MIC) in- vestigations have addressed the impact of pure or mixed cul-
ture bacterial biofilms on corrosion behavior of iron, copper, aluminium and their alloys. The main types of bacteria associ- ated with metals in terrestrial and aquatic habitats are sul- fatereducing bacteria (SRB), sulfur-oxidising bacteria, ironox- idising/ reducing bacteria and manganese-oxidising bacteria secreting organic acids and slime. These organisms typically coexist in naturally occurring Biofilms, forming complex con- sortia on corroding metal surfaces.Once the microorganism forms a biofilm on a material’s surface, a microenvironment is created that is dramatically different from the bulk surround- ings. The biofilm with microorganisms’ metabolic reactions attributable to metallic corrosion involve sulfide production, acid production and metal oxidation and reduction. SRB and Sulphate oxidizing bacteria (SOB) generate sulfide and Sul- phate production leads to health and safety problems, envi- ronmental hazards and severe economic losses. The role of these bacteria in the pitting corrosion of various metals and their alloys in both aquatic and terrestrial environments under the anoxic as well as oxygenated conditions [3]. The biofilm producing organisms such as Pseudomonas aeruginosa, Pseudo- monas fluorescence and E.coli and the experimental organisms Desulfuvibrio and Thiobacillus thioparus are isolated from the water distribution system of study area. This study focused on
the activity of consortium of SRB and SOB on EPS was investi-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 735
ISSN 2229-5518
gated.
2.1 Material Methods
2.2 Study area and sample collection
The study was conducted in Bangalore University, Jnanabharathi campus consist of 50 different faculties which was around 1200 acres located in south Karnataka. The water samples were collected from the bio park drinking water distribution system using sterilized plastic carboys bottle. Immediately all the samples were transferred to laboratory for experiments.
2.3 Isolation of organism
The principle study of biofilm forming microorganism such as Pseudomonas aeruginosa, Pseudomonas fluorescence, E.coli and Iron oxidizing organisms such as Desulfuvibrio sp., Thiobacillus thioparus are isolated from the water distribution system fol- lowing method of Bergey’s manual and APHA, 2005[4] [5]. All the necessary bio chemical test was carriedout to confirm these organisms and culture was regenerated and preserved at 37° C for there experiment.
2.4 Remnant preparation from galvanized iron pipe
Specimen of galvanized pipe coupons of (1.5cm × 1.5cm) were polished on progressively finer silicon carbide papers to a final grit size of 1000. After polishing they were rinsed in distilled water and then in acetone for degreasing. Later, the specimens were immersed in 70% ethanol for 4hrs.All the coupons and necessary glassware used for the study was au- toclaved at 121° C for 30 minutes and later dried in a hot air oven [6].
2.5 Experimental setups
For this study six experimental setups was arranged in differ- ent manner that two sets for control and rest of them for test.
300ml of water sample was collected in a series of sterilized coni- cal flask and add 10ml of nutrient broth (13g/1000ml) and approx- imately 3 to 5 g (2 × 4 cm) galvanized iron remnant was exposed into the flask and autoclaved at 121° C for 30 minutes. The Pseu- domonas aeruginosa, E-coli were inoculated together in all 10 flasks at 27°C and similar procedure was carriedout for combination of Pseudomonas fluorescence, E-coli together. All the experimental set up was maintained at 37° C in shaker incubator.
2.6 Inoculation of Desulfuvibrio sp., Thiobacillus thioparus:
After biofilm formations the isolated Desulfuvibrio (4×106
————————————————
• Sivasakthivel.S, Ph.D Scholar, Dept.of.Environmental Science, Bangalore
University,Karnataka,India, PH-+918147187948.
E-mail: sakthisiva1982@yahoo.com
cells/ml) were inoculated in to setup of Pseudomonas aerugi nosa, E-coli, control were not inoculated. Like wise Thiobacillus thioparus (4×106 cells/ml) were inoculated in to setup of Pseu- domonas fluorescence and E-coli except control.
2.7 Extraction and estimation of extra polymeric substance
All the coupons were removed from experimental setup and the extra polymeric substance was extracted by EDTA (2%) method. 20ml of EDTA (2%) add to the flask containing galvanized iron remnants. Biomass (0.6g ts.dry wt) from the biofilm was washed and centrifuged twice with 60ml deion- ised water. The precipitate was collected and dissolved in
100ml deionised water in a vortex blender. To 30ml sample
was added 30ml EDTA (2%) and the mixture was left at 4° c. the electric power was applied when necessary. The suspen- sion mixture was agitated for 3 minutes every 30 minutes to avoid the possible precipitation of biomass. After extraction the suspension mixture was centrifuged the supernatant fil- tered through a 0.22µm cellulose acetate membrane to remove the residual cells [7].
2.8 Purification of polysaccharides
The initial PH of the extracts was 7.17 and the adjustment of other PH values performed by the addition of 1M NaoH or 1M Hcl. The PH was adjusted to a new value and after 2h the pre- cipitate was recovered by vacuum filtration through a mem- brane of 0.45µm. The filtrate was assayed for total protein con- tent and polysaccharides [7].
2.9 Estimation of protein
The content was assayed by burette method. 1ml of extract was taken for test the standard protein was prepared using BSA (5,10,15,20,25 mg). The total protein was estimated by calorimetrically at 540nm against 1ml of distilled water as blank [8].
2.10 Estimation of polysaccharide
The polysaccharide was analyzed by the phenol – sulfuric acid calorimetric method using standard glucose (concentration
5.10,15,20,25 mg). 1ml of extract was taken for test. The total polysaccharide was estimated by calorimetrically at 490nm. Against the blank of 1 ml distilled water [9].
3 Results
After 45 days the EPS was developed on all the experimental setups including test and control. The experimental organisms Desulfovibrio sp., (SRB) and Thiobacillus thioparus (SOB) was inoculated and the EPS was predicted every in a week and compared with control.
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 736
ISSN 2229-5518
I Projection of EPS Enhanced by Desulfovibrio (SRB) along with set up of Pseudomonas aeruginosa and E-coli together
Significant level of EPS were observed after introduced SRB in to the experimental set ups. Initially, 19µg of polysaccharides and 16 µg of proteins were excreted. After the inoculation of this experimental organism, the greatest level of EPS was ob- served in the system of test which was found to be 44µg of polysaccharides and 31 µg of protein, where as in control found to be 22 µg of polysaccharides and 19 µg of proteins on
52th days (Table-1). Like wise, 56 µg of polysaccharide and 39
µg of proteins were projected on 59nd days, but in control 22
µg of polysaccharide and 19 µg of proteins were observed. In
the same way, 61 µg of polysaccharide and 46 µg of proteins was anticipated on 66th day, where as control 32 µg of polysac- charide and 26 µg of proteins was observed. Ultimately, the maximum intensity of EPS was projected on 72th day which
was found to be 65 µg of polysaccharides and 51 µg of proteins was estimated from the test system, where in control 39 µg of polysaccharide and 30 µg of protein was observed.
Table 1: Range of EPS projected before and after inoculation of SRB, SOB.
No of Da ys | EPS of Pseu- domonas Aeru- ginosa and E- coli together (Control- I) | Action of Desulfo vibrio sp., (SRB) | EPS of Pseudomo- nas fluores- cence and E-coli to- gether ( control - II) | Action of Thio- bacillus thiopa- rus (SOB) | ||||
No of Da ys | Poly- sac- cha- rides (µg) | pro- tein (µg) | Pol- ysac cha- ride s (µg) | pro- tein (µg) | Pol- ysac cha- ride s (µg) | pro- tein (µg) | Poly- saccha- rides (µg) | pro- tein (µg) |
30 | ND | ND | Not inoc- ulated | ND | ND | Not inoculated | ||
45* | 19 | 16 | Inoculat- ed | 23 | 20 | Inoculat- ed | ||
52 | 22 | 19 | 44 | 31 | 26 | 22 | 24 | 18 |
59 | 27 | 23 | 56 | 39 | 32 | 26 | 21 | 15 |
66 | 32 | 26 | 61 | 46 | 38 | 31 | 19 | 11 |
72 | 39 | 30 | 65 | 51 | 43 | 38 | 15 | 10 |
ND- Not Detected, SRB- Sulphate Reducing Bacteria
II Action of Thiobacillus thioparus on EPS Produced by
Pseudomonas fluorescence and E-coli together:-
After 45 days, the slimy layer of EPS was observed on the coupon containing all the flasks. The quantity of EPS were analyzed before and after inoculation of experimental organ-
ism (T.thioparus) and compared with EPS of control system. The in situ results of action of Thiobacillus thioparus on EPS are shown in table 1.
Initially, 23µg of polysaccharide and 20 µg of protein were extracted on 45th day. After inoculation, 24 µg of polysaccha- rides and 18 µg of protein were estimated on 52nd day, where in control 26 µg of polysaccharides and22 µg of proteins was quantified. The result indicates the level of EPS was quietly reduced after introduced the Thiobacillus thioparus. Similarly,
21 µg of polysaccharides and 15 µg of proteins were estimated on 59th days, where in control the maximum quantity of EPS in the control system. Like wise, further the level of EPS was re- duced by 19 µg of polysaccharides and 11 µg of proteins on
66th day(Figure 1). The most part of EPS were consumed by
SOB on end of 72nd days which was found least quantity about
15 µg of polysaccharides and 10 µg of proteins.
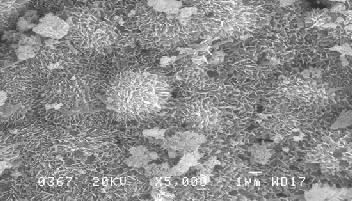
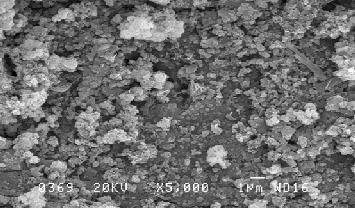
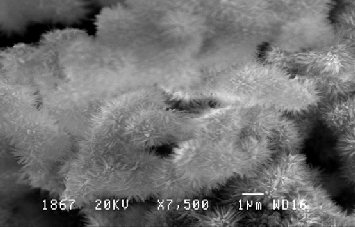
Figure 1: SEM Image of galvanized iron coupon showing tabular hexagonal crystals and spongy globules due to SRB’s colonization:-

III Efficient excretion EPS by Pseudomonas fluorescence
with the combination E-coli together
From the EPS result of Pseudomonas fluorescence along with the combination of E-coli illustrated that Ps. Fluorescence excretes the maximum level of EPS when compare to the combination of Pseudomonas aeruginosa and E-coli ( figure-5). Initially, 43 µg of EPS was projected by Ps. fluorescence on 45th day, whereas Pseudomonas aeruginosa excreted the 35 µg along with the com- bination of E-coli. On 52nd day the level of EPS was found to be
48 µg greater than before, where as 41 µg of EPS were quanti- fied in the system of Pseudomonas aeruginosa and E-coli togeth- er. Ever in a week, the furthermost level of EPS was extracted form the experimental system of Pseudomonas fluorescence and E-coli together (Table-2). At the end of the day, 81 µg of EPS was counted, where as in Pseudomonas aeruginosa- E-coli secret- ed around 69 µg. In a straight line, the results point out the Pseudomonas fluorescence has more competent than Pseudomo-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 737
ISSN 2229-5518
fovibrio
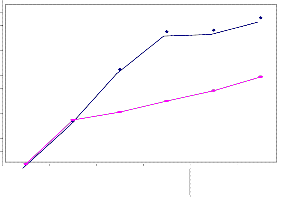
PROJECTION OF EPS ENHANCED BY DESULFOVIBRIO
After Inoculation
Control set
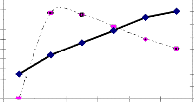
The EPS test results of Desulfovibrio at different period shows that action of Desulfovibrio sp., on EPS produced by combina- tion of Pseudomonas aeruginosa and E-coli together, it was ob- served that the experimental organism enhance the EPS secre- tion along with Pseudomonas aeruginosa and E-col(Figure-1).
The greatest level of EPS was observed in every 7 days of in- terval than the control(Figure:2). Directly, it demonstrate that desulfovibrio enhance biofilm producing bacteria. Sulphate reducing bacteria were present in significant quantities within the biofilm, even after exposure to free chlorine or mono chlo- rine[10].
The statistical graph plot between the test and control repre- sent that, the test slope randomly raised than control slope in every time of day. This is the direct evidence that confirm the Desulfovibrio(SRB) itself produce the EPS along with Pseudo- monas aeruginosa and E-coli. Sulphate reducing bacteria are an aerobics that have been found to be involved with numerous microbial influenced corrosion by producing biofilms[11].
After inoculation of Thiobacillus thioparus , the EPS results shows sulphur oxidizing organisms exploit the EPS gradual- ly(Figure 3).The quantity of EPS gradually diminished in eve- ry in a week was observed(Figure-4). In other hand the Thio- bacillus spp was populated in every in a week not shown this table.. Thiobacillus spp., are the bacteria most widely involved in such leaching operations[12][13] [14]. A Directly, it demon- strate the sulphur oxidizing bacteria ( Thiobacillus sp,.) may suspend the EPS or utilsed for their populations.. Sulfur oxi- dizing bacteria may be of use in determining the food web relationship of other ecosystem that have at least a partially chemoautrophic base [15]. The combination study of P.aeruginosa and P. flouresence with E-coli reveals that P. flouresence produced more EPS than the P.aeruginosa. Pseudo- monas spp are biofilm bacteria plays important role for initiat- ing Biological mediated corrosion in the aqueous environ- ment. Efficiency of EPS secretion is varies in species level in within the genera [16].
Figure 2: Represents the level of EPS Enhanced by Desul-
No of Days
Pseudomonas flourescence and E-coli :-
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 738
ISSN 2229-5518
The population of Thiobacillus thioparus Gradually increased
50
45
40
35
EPS in 30
25
20
15
10
5
0
EPS
Microbial
Growth
0 45 52 59 66 72
No of Days
16
14
12 106 cells/ml
10
8
6
4
2![]()
![]()
![]()
0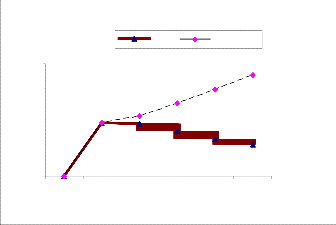
Figure 9: Efficiency of EPS projection of Pseudomonas fluorescence with the Combinations of E-coli.
ACTION OF THIOBACILLUS THIOPARUS ON EPS
TEST CONTROL
90
80 con trol
70
60
50
40
Thiobacillus
30
20 spp,.
10
0
EPS
in
µg
EFFICIENCY OF Psudomonas flourescence WITH THE COMBINATION OF of E-coli
![]()
350
0
0
0
0
100
50
0
45 52 59 66 72
No of Days
Pseudomonas aeruginosa and
E-coli together
Pseudomonas Fluorescence and
E-coli together
De cline of EPS produce d by a ctivity of Thiobacillus spp,.
Biocorrosion take place in water and terrestrial habitats that
50 W- No of weeks
45
40 - SOB
35 Inoculated
30
25
20
15
10
5
0
differ in nutrient content, temperature, pressure and pH. It results from the presence and physiological activities of mi- crobial consortia on the metallic surfaces. Pseudomoans
W2
flouresence had more efficient for secretion of EPS than the
W3
Pseudomonas aeruginosa. From the detailed study of SRB and
SOB on Biofilms revealed that SRB’s are enhances Pseudomonas
aeruginosa and E-coli for secretion of Biofilm through the sym-
biotic relation. Concurrently, SOB’s are utilizing the Polysac-
charides as a carbon source available in the Biofilm. The com-
0 20 40 60 8
No of Da ys
parative study of SRB and SOB on Biofilm directly point out
that consortium of these microbes undergoes the cycling pro-
cess of enhancement and utilization of Biofilm in the aquatic
metal surface. The cyclic phenomenon repeatedly takes place
in metal surface in aquatic environment throughout the life
span. From this effort to understand of cyclic phenomenon
between the SRB and SOB on Biofilm advice that SRB’s and
Pseudomonas aeruginosa and E-coli growth to be control to
IJSER © 2013 http://www.ijser.org
International Journal of Scientific & Engineering Research, Volume 4, Issue 6, June-2013 739
ISSN 2229-5518
prevent the MIC on metal surfaces in the aquatic environment.
The authors wish to thank University Grant Commission (UGC). New Delhi. This work was supported in part by a grant from UGC Major Research project.
[1] H. Heuklekian., Heller.A, “Relation between food concentration and surface for bacterial growth”, J. Bacteriol.. 40: 547-58, 1940.
[2] E.E.Geldriech , “Microbial processes in water supply distribu- tion,”Seminar presentation at the American Association for Microbi- ology,FL.May 14,1980.
[3] Maniano Tumaru, Maria Dragomir,” The involment of microbiologi-
cally induced corrosion in nuclear power plants systems”. WEC re- gional energy forum. Neptum 15-19R. Nicole, "The Last Word on De- cision Theory," J. Computer Vision, submitted for publication. (Pend- ing publication), 2008.
[4] Bergey’s Mannual,” Systematic bacteriology”, Vol-2, 2 nd edi- tion.2005.
[5] APHA,”standard methods for the examination of water and waste water”, 21st edition ,2005.
[6] Congmin Xu, Yaoheng Zhang, Guangxu Cheng, Wensheng Zhu, “Pitting corrosion behavior of 316L stainless steel in the media of sulphate- reducing and iron-oxidizing bacteria, Materials Characetisation, MTL
06170”,Vol.1, No.1,2007.
[7] R.Olivera, F. Marques and J.Azeredo,” Purification of plysaccharides from a biofilm matrix by selective precipitation of proteins”, Biotech- nology techniques. vl-13. p 391-393,1999.
[8] O.H. Lowry, Rosenbrough, N.J, Farr, A.L, Randall,R.”Proteing meas-
urement with the folin phenol reagent”,J Biol Chem,vol.193,pp.265-
275,1951.
[9] Michel Dubois, K.A.Gilles, J.K.Hamilton, P.A.Rebers, and Smith,”
colorimetric method for determination of sugars and related sub- stance”, Vol-28. no-3.p360-355,1959.
[10] W.Mark, Lechevallier, Cheryl D. Cawthon, And Ramon G. Lee,” Inactivation of Biofilm Bacteria”, Applied And Environmental Mi- crobiology ,Vol. 54, No. 10, p. 2492-2499,1988.
[11] David L. Douglas ,” Fundamental Aspects of Iron Corrosion; Corrosion and Wear Handbook, for water-cooled reactors”, United States Atomic Energy Commission McGraw-Hill Book Company, Inc. New York, London, 1951.
[12] Lundgren,D.G, Silver. M, “Ore leaching by bacteria”, Annu.rev. Mi-
crobiol.Vol. 34, 263-283,1980.
[13] W.J.Inglede, “ Thiobacillus ferroxidans; The bioenergetics of an acidophilic chemo- lithotroph”, Biochem. Bio physics. Acta 683.p-89-117,1982.
[14] S.R.Hutchins , Davidson M.S, Brierley C.L, “ Microorganisms in reclamination of metals”, Annu. Rev. Microbiol. 40,p 311-336,1986.
[15] Luminitha Vlasceanu, Radu popa and Brain Kinkle,” characterization of Thiobacil- lus thioparus LV 43 and its distribution in a chemoautotrophically based ground waster ecosystem”, Applied and environmental microbiology, vol- 63,no-8,p 3123-
3127,1997.
[16] P.J.Antony, Shobhana Chongdar,Pradeep Kumar and R.Raman,” Corrosion of 2205 duplex stainless steel in chloride medium containing sulfate reducing bacte- ria”,Electrochemica acta. Vol.52,No.39,85-94,2007.
IJSER © 2013 http://www.ijser.org